US Invests $2.8 Trillion to Expand Domestic Production
China Bans Use of Foreign Medical Devices
Impact on Korea?
Bio: "Limited Impact"... Could Be a Blessing in Disguise
Medical Devices: "China Accelerates Domestic Substitution... Need to Widen Technology Gap"
[Asia Economy Reporter Chunhee Lee] The high-tech supremacy battle between the United States and China is expanding beyond semiconductors into the bio and medical device sectors. As the Biden administration in the U.S. plans to invest more than $2 billion (approximately 2.788 trillion KRW) to foster its domestic bio industry, China is also excluding foreign-made medical equipment from hospitals, intensifying the competition for future growth industries between the two countries.
For South Korea's bio and medical device industries caught in the middle of the U.S.-China conflict, finding an appropriate response strategy has emerged as a critical task. While there is hopeful analysis that the bio industry could experience a sort of turning point, the medical device sector faces the risk of losing the key Chinese market without securing technological competitiveness.
Bio Industry "Limited Impact"... Could Even Become an Opportunity to Enter the U.S. Market
In the bio industry, it is widely expected that global big pharma companies are increasing their share of Contract Development and Manufacturing Organizations (CDMO) themselves, and due to the high entry barriers of the industry, the impact of the U.S. executive order is not expected to be significant.
Celltrion Group issued a statement saying, "After reviewing the details of the executive order, we judge that the impact on Celltrion Group so far is minimal." Researcher Jung Yookyung from Shin Young Securities explained, "The immediate impact of this executive order will be limited. CDMOs are used to secure multiple production bases and outsource a considerable volume, so it is not easy to switch to production solely within the U.S."
Jung Yuntaek, head of the Pharmaceutical Industry Strategy Institute, also said, "Unlike electric vehicles, it is difficult to implement active subsidy policies for biopharmaceuticals, so the damage to the domestic industry will not be significant," adding, "Since the U.S. has expressed its intention to actively promote industrial development, companies considering entry into the U.S. market may see this as an active investment opportunity." Samsung Biologics is reportedly reviewing new factory sites in California and Washington states, and Celltrion also stated, "We will closely examine incentive systems and actively consider securing direct production facilities in the U.S. if deemed advantageous."
Medical Devices: Risk of Being Left Behind Without Bridging the Technology Gap
On the other hand, the medical device industry is widely expected to suffer significant damage if China's policy to restrict foreign medical devices materializes. Not only advanced medical device countries such as the U.S., Japan, and Germany but also domestic companies accelerating their entry into the Chinese market are expected to be affected.
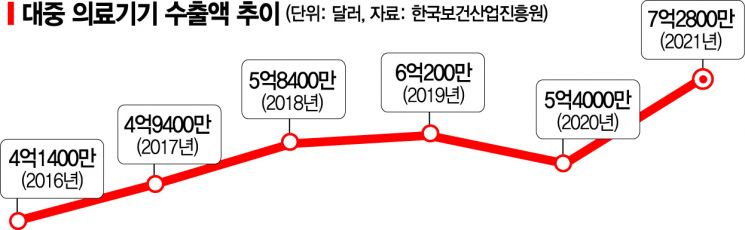
According to statistics from the Korea International Trade Association, as of last year, China's medical device market size reached 192 trillion KRW, growing to rival the U.S., which has the world's largest medical device market. The export share from South Korea is also significant. According to the Korea Health Industry Development Institute, medical device exports to China last year amounted to $728 million (approximately 1 trillion KRW), ranking second after the U.S. ($916 million). Although exports slowed somewhat in 2020 due to the COVID-19 pandemic, they have since increased by 34.9% year-on-year, maintaining growth. In particular, exports have increased centered on key products such as implants and ultrasound diagnostic devices.
However, there are concerns that this growth trend could sharply decline if foreign medical devices are restricted. An industry insider said, "Unlike pharmaceuticals, medical devices are closer to manufactured goods," adding, "Since the Chinese government has emphasized increasing the localization rate of core medical device components for several years, import substitution is likely to occur rapidly."
In this regard, it has been pointed out that domestic medical device companies will find it difficult to survive unless they maintain their technological gap. Another industry insider said, "Medical devices requiring high technology such as ultrasound and magnetic resonance imaging (MRI) still have foreign companies holding an 80% market share in China," and predicted, "For high value-added products like ultrasound, which are major export items to China, the immediate impact will not be significant."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















