#1, Silicon Anode Material for High-Capacity, High-Speed Charging, "Breaks Easily When Cold"
#2, Observation of Liquid-State Materials at Atomic Level... Proposal of Real-Time TEM Technique
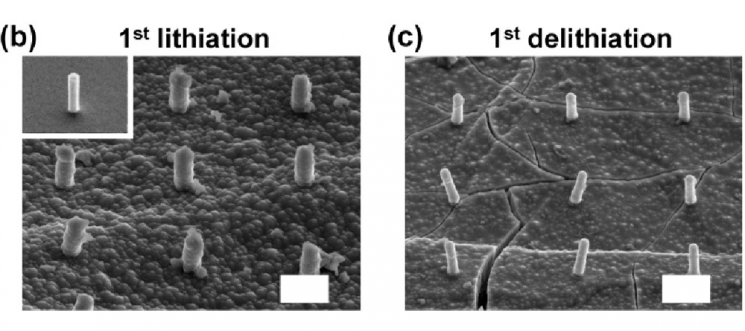
 A research image showing severe destruction of silicon nanopillars during charging and discharging at low temperature (right) compared to charging and discharging at room temperature (left).
A research image showing severe destruction of silicon nanopillars during charging and discharging at low temperature (right) compared to charging and discharging at room temperature (left).
 A research illustration showing silicon nanopillars swelling when charged (left) and returning to their original shape when discharged (right).
A research illustration showing silicon nanopillars swelling when charged (left) and returning to their original shape when discharged (right).
[Asia Economy Yeongnam Reporting Headquarters, Reporter Kim Yong-woo] Remarkable research that accelerates the development of next-generation batteries has been consecutively released in Korea.
This research, which has caught the attention of the global academic and industrial communities, consists of two important papers on the development of high-capacity and fast-charging batteries. They cover the study of silicon, a candidate material for next-generation batteries, and a transmission electron microscopy (TEM) technique to discover new battery materials.
The research team led by Professor Lee Hyun-wook of the Department of Energy and Chemical Engineering at Ulsan National Institute of Science and Technology (UNIST) recently published two papers consecutively in the international journal Nano Letters.
The first paper analyzes the charge-discharge characteristics of silicon, considered a next-generation anode material, at different temperatures, and the second introduces a new technology for real-time observation of liquid materials using transmission electron microscopy.
The first paper on silicon properties was conducted jointly with Professor Lee Seok-woo’s team at Nanyang Technological University in Singapore and supported by the Korea Energy Technology Evaluation and Planning (KETEP) energy workforce development program. (Paper title: Temperature-dependent fracture resistance of silicon nanopillars during electrochemical lithiation)
Silicon has about ten times the capacity of graphite, the commercialized anode material. Because of this, it has been considered a promising candidate for high-capacity battery materials, but it has a critical drawback.
Repeated charging and discharging cause silicon to expand, leading to the destruction of individual particles and electrons. Additionally, when a solid electrolyte interphase forms along the fractured surfaces, lithium-ion transport slows down, which is also problematic.
Professor Lee Hyun-wook said, “To use silicon as a next-generation anode material, securing structural stability against volume expansion is the top priority,” adding, “This study aimed to find new structural stability of silicon by analyzing the volume expansion and fracture behavior of silicon anode materials at different temperatures.”
To find improvements, the research team fabricated silicon nanopillars of various diameters on three types of single-crystal silicon wafers with different orientations using an electron beam.
They assembled battery cells centered on the nanopillars and observed the electrochemical reactions between lithium and the silicon wafers during charge and discharge.
The results showed that each nanopillar exhibited different volume expansion behaviors after lithium charging depending on the crystallographic orientation of the silicon wafer.
Seojeong Yeom, the first author and an integrated master’s and doctoral course researcher at UNIST’s Department of Energy and Chemical Engineering, explained, “Depending on the characteristics of the silicon crystal plane, expansion occurred in two, four, or six directions, showing different properties in low-temperature and above-room-temperature environments.”
Yeom added, “At high temperatures, the directionality of volume expansion decreases, while below 0°C, the directionality increases, causing the nanopillars to fracture more easily.”
The team also analyzed the fracture behavior of silicon nanopillars subjected to lithium charge-discharge cycles in low-temperature environments below -20°C.
While 300-nanometer-diameter silicon nanopillars remained relatively stable after two lithium charges at room temperature, they were found to be 100% fractured under low-temperature conditions.
Professor Lee Hyun-wook said, “Charging and discharging in cold winter environments can cause volume expansion and fracture in silicon anodes,” adding, “Further research is needed to elucidate the mechanical behavior of silicon anodes at low temperatures and develop methods to mitigate fracture.”
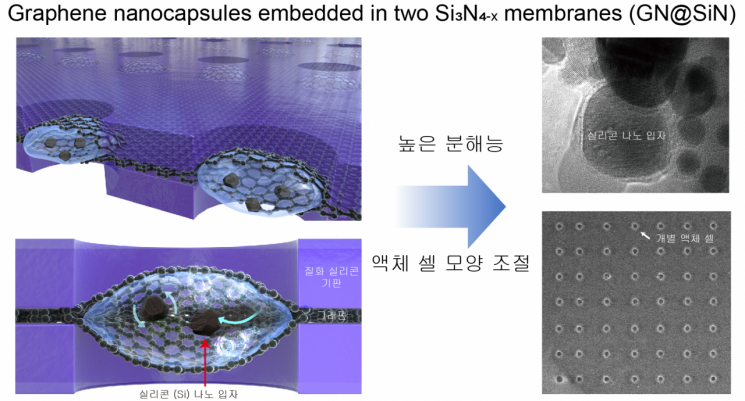 Schematic diagram of a liquid capsule made by coating single-crystal graphene on a silicon nitride film.
Schematic diagram of a liquid capsule made by coating single-crystal graphene on a silicon nitride film.
The second paper focuses on advancing the TEM technique, a tool for discovering next-generation battery materials.
They developed a new method using single-crystal graphene to observe the movement of liquid-state materials at the atomic level.
This research was jointly conducted by Professor Jin Sung-hwan of UNIST’s Department of Energy and Chemical Engineering and Rodney Ruoff, director of the IBS Center for Multidimensional Carbon Materials and a distinguished professor at UNIST. (Paper title: Using Single-Crystal Graphene to Form Arrays of Nanocapsules Enabling the Observation of Light Elements in Liquid Cell Transmission Electron Microscopy)
TEM is a microscope that observes materials by irradiating them with an electron beam, allowing magnification thousands of times higher than optical microscopes.
When the subject is liquid, it must be kept in a high vacuum to prevent evaporation. Therefore, liquids have been enclosed using about 50-nanometer-thick silicon nitride membranes or single-atom-thick graphene to analyze internal materials.
However, the thickness of silicon nitride membranes obscures the subject, making it difficult to obtain high-resolution images. When using graphene, the shape, position, and size of the liquid-trapping areas vary, limiting consistent observation conditions.
This study developed a new liquid capsule to solve these problems.
They uniformly drilled holes of several hundred nanometers in the desired silicon nitride membrane location and synthesized and coated it with single-crystal graphene.
When liquid is placed between two membranes and overlapped, the graphene membranes covering the holes bulge up and down, trapping the liquid between the graphene layers. (Figure 3)
Professor Jin Sung-hwan said, “Using single-crystal graphene, which is 100 times thinner and more than three times stronger than silicon nitride membranes, we maximized the resolution of TEM images by effectively trapping the liquid.”
He explained, “The size, position, and shape of the liquid capsules can be freely adjusted, allowing multiple observations of materials under identical liquid conditions.”
Using the newly developed liquid capsules, the thickness of the liquid through which the electron beam passes is much thinner than before, making it advantageous for observing light elements, polymers, and viruses.
Professor Lee Hyun-wook said, “We will clarify the liquid-phase synthesis process and motion mechanisms of light compounds that have not been observed before, accelerating and strengthening battery material development.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.






![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















