FDA Approval Decision on the 9th
First Domestic New Drug Approved by FDA in 3 Years and 6th Overall
Factory Inspection Issues Already Resolved
US Partner Also Fully Equipped with Sales Network
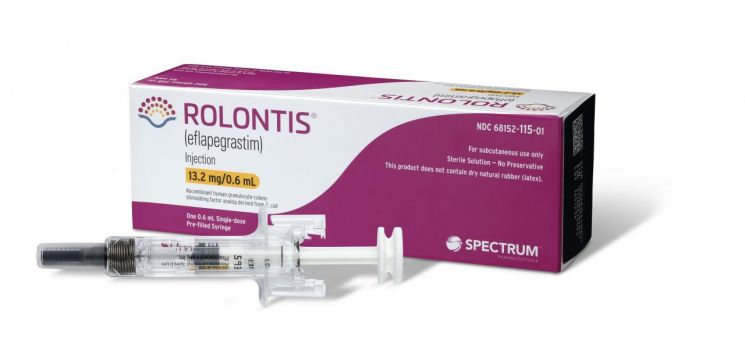
[Asia Economy Reporter Lee Chun-hee] The deadline for the U.S. approval decision of Hanmi Pharmaceutical's neutropenia treatment drug 'Rolontis' is fast approaching. Attention is focused on whether the history of FDA approval for Korean new drugs, which has been dormant since 2019 for three years, will be revived.
According to related industries on the 4th, the fate of Rolontis is expected to be decided on the 9th (local time). This is because, according to the U.S. 'Prescription Drug User Fee Act (PDUFA),' the FDA set the approval review deadline as the 9th of this month upon receiving the Biologics License Application (BLA) for the biopharmaceutical product.
If approval is granted on the 9th, it will be the first Korean new drug to receive FDA approval in three years since November 2019 with SK Biopharm's 'Xcopri,' and the sixth overall. Following FDA approvals for ▲LG Chem's 'Pactive' ▲Dong-A ST's 'Cefextro' ▲SK Chemicals' 'Abstila' ▲SK Biopharm's 'Sunosi' and Xcopri, Rolontis will continue the legacy.
For Hanmi Pharmaceutical, it is significant as it will be the first global market approval among biopharmaceuticals applying its proprietary platform technology 'Lapscovery.' Lapscovery is a technology that extends the duration of drugs in the body, overcoming the short half-life, which is considered a drawback of biopharmaceuticals.
The Biggest Hurdle 'Factory Inspection' Already Overcome
This is the third attempt for Rolontis to gain U.S. approval. Spectrum, the U.S. partner company that licensed Rolontis in 2012, applied for a Biologics License Application (BLA) to the FDA in 2018 but voluntarily withdrew the application after the FDA requested additional data. The following year, they reapplied for the license, but in August last year, they received a Complete Response Letter (CRL) from the FDA and reapplied for approval again in March this year.
Since the issues with the manufacturing facility have been resolved, expectations for approval success are growing. The FDA inspection of the Pyeongtaek bioplant, where the Rolontis active pharmaceutical ingredient is produced, was conducted in June, delayed due to the COVID-19 pandemic and other factors. Hanmi Pharmaceutical explained, "The inspection was completed without major findings."
Spectrum recently finalized the U.S. launch name of Rolontis as 'Rolvedon' and has completed preparations for launch, including recruiting sales and marketing personnel to cover the entire U.S. market.
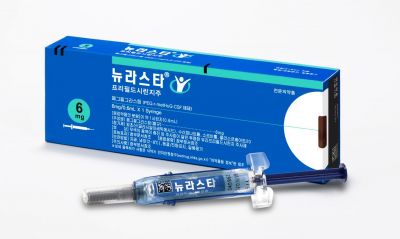
Can It Catch the Blockbuster 'Neulasta'?
Rolontis is a treatment for neutropenia. Neutrophils play a role in defending against bacterial and fungal infections within white blood cells. However, cancer patients frequently experience neutropenia, a severe reduction in neutrophils, during chemotherapy, leading to significantly weakened immunity. Rolontis is used for the treatment or prevention of neutropenia. It was already approved and marketed domestically in March last year as the 33rd Korean new drug.
The global market leader in neutropenia treatment is Amgen's 'Neulasta.' It is a blockbuster drug with annual sales of about $5 billion (approximately 6.815 trillion KRW). In Korea, it has maintained the number one position for several years, generating sales of 24.4 billion KRW last year. However, sales have been declining recently due to the launch of various biosimilars (biopharmaceutical generics).
A Hanmi Pharmaceutical official said, "Rolontis is significant as Hanmi Pharmaceutical's first global new drug," adding, "We will focus the group's capabilities to ensure that we achieve tangible results in the neutropenia treatment market, which forms a market of about 3 trillion KRW in the U.S. alone."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















