Synthetic Antigen Vaccines with Relatively Fewer Side Effect Concerns
SK Bioscience Acts as CDMO Domestically
[Asia Economy Reporter Lee Chun-hee] Novavax's COVID-19 vaccine has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA).
On the 13th (local time), the FDA announced that after reviewing the relevant data on the Novavax vaccine, it determined that the benefits outweigh the potential risks for individuals aged 18 and older, and thus approved emergency use for those 18 and older. It is the fourth COVID-19 vaccine approved in the United States, following Pfizer, Moderna, and Janssen (a subsidiary of Johnson & Johnson). The fourth COVID-19 vaccine is now in use.
Robert Califf, FDA Commissioner, stated, "Additional COVID-19 vaccine authorizations expand the vaccine options available for COVID-19 prevention," adding, "It will provide another option for U.S. adults who have not yet been vaccinated against COVID-19." He further said, "Vaccines remain the best measure to prevent severe illness," and "encourages those who have not yet received a COVID-19 vaccine to consider getting vaccinated."
Earlier, the Ministry of Food and Drug Safety (MFDS) in South Korea decided to grant product approval on the condition that Novavax submit the final clinical trial report in January. As of the 12th, a total of 590,870 doses have been administered domestically. Notably, nearly half of these, 291,763 doses, were fourth booster shots.
This is because even those who previously received other vaccines can choose to receive their third or fourth booster shots with the Novavax vaccine if they wish. Developed as a recombinant protein vaccine, a method traditionally used for vaccines such as influenza, hepatitis B, and cervical cancer, it is analyzed to have relatively lower concerns about side effects, leading to increased preference.
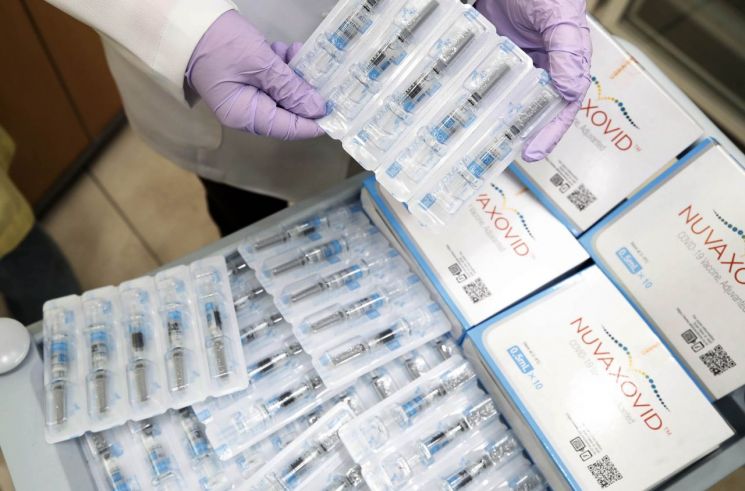
Currently, SK Bioscience is contract-developing and manufacturing (CDMO) the Novavax vaccine domestically. While the Novavax vaccine is mostly distributed overseas in vials containing 10 doses, SK Bioscience has conducted additional research and development to produce a 'prefilled syringe' form containing a single dose per syringe. This reduces contamination risks during separate dilution or aliquoting processes and improves vaccination convenience.
However, the Novavax vaccine distributed in the U.S. is likely to be primarily supplied by the Serum Institute of India (SII), the world's largest vaccine manufacturer. This FDA emergency use authorization was based on inspections and evaluations of SII.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.