The Biggest Difference is the Form of Treatment Delivery
DTx, 'Software Medical Devices'... Must Not Depend on Hardware
Electronic Drugs Must Be Used with Hardware
Depression Electronic Drug 'Mind Steam', Recently First Prescribed
Can Electronic Drugs Overcome Dementia, Which Still Has No Medication?
[Asia Economy Reporter Lee Chun-hee] Recently, Hanmi Pharm and KT decided to make a joint investment in Digital Pharm, which develops 'Digital Therapeutics (DTx)' and 'electroceuticals,' bringing both DTx and electroceuticals into the spotlight simultaneously. While DTx is a frequently mentioned concept, especially after Pear Therapeutics in the U.S. launched the first FDA-approved DTx 'reSET' and got listed on the U.S. stock market, electroceuticals remain a somewhat unfamiliar concept. At first glance, these two treatments may seem similar, but how do they differ?
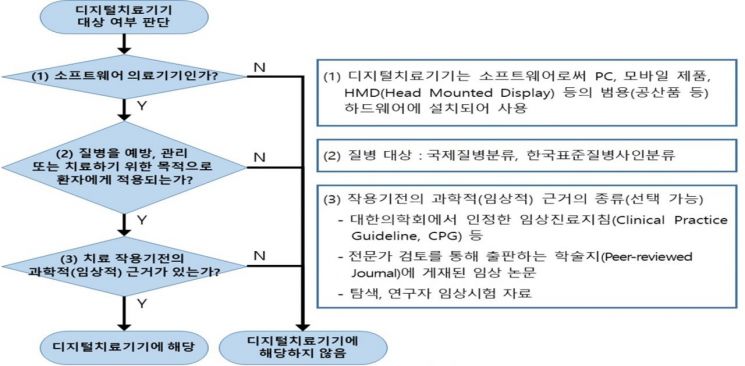
When discussing 'digital healthcare,' DTx and electroceuticals are often grouped under the same category. However, electroceuticals and DTx are distinctly different concepts. First, the Ministry of Food and Drug Safety (MFDS) defines DTx (here referred to as 'digital therapeutic devices') as 'software as a medical device (SaMD) that provides evidence-based therapeutic interventions to patients to prevent, manage, or treat medical disorders or diseases.' SaMD stands for 'Software as a Medical Device,' meaning software functioning as a medical device. The MFDS further clarifies that SaMD is 'a medical device composed solely of software that is independent and not dependent on hardware, with functions aligned with the intended use of the medical device.'
This point is the key that separates DTx from electroceuticals. Typical DTx is delivered via applications (apps) installed on smartphones or tablets or accessed through web services on computers. While hardware is necessary to run these apps or web services, it is not considered an essential component of DTx itself.
On the other hand, electroceuticals are not composed solely of software. They involve invasive actions such as wearing a separate device on the body to apply electrical stimulation to the brain or nerve cells. Since it is difficult to perform their essential functions without accompanying hardware, electroceuticals can be seen as 'dependent on hardware.'
Some critics even question the term 'electroceuticals' itself because the current Pharmaceutical Affairs Act defines drugs as 'not being instruments, machines, or devices.' While this might cause some confusion when called a therapeutic agent, the official term used by the MFDS is 'therapeutic device,' which reduces misunderstanding. However, since the term 'drug' is directly used for electroceuticals, there are concerns about this nomenclature.
It is also pointed out that the lack of clear domestic regulations related to electroceuticals contributes to this confusion. However, considering that the MFDS recently defined DTx as 'digital therapeutic devices,' this situation could be somewhat overcome through future government efforts.
Recently, various electroceuticals have been developed or commercialized both domestically and internationally. Ybrain recently announced the first non-reimbursed prescription of the electroceutical for depression, 'Mind Stim.' The prescription was given to a female patient preparing for pregnancy who was experiencing depression and was concerned about drug treatment.
Ybrain is also developing electroceuticals for mild dementia. They are currently conducting confirmatory clinical trials nearing commercialization and plan to obtain MFDS marketing approval within this year or early next year at the earliest. Since dementia treatments have effectively reverted to a state of no available drugs after the first FDA-approved drug, Biogen's 'Aduhelm' (generic name 'aducanumab'), faced commercialization setbacks, there is growing interest in whether a new path to overcoming dementia will open.
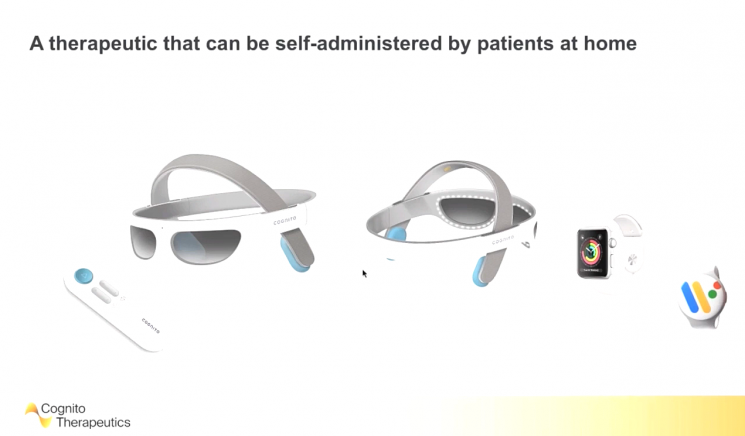
Overseas, companies like Cognito Therapeutics are attempting to develop electroceuticals under the motto 'Electricity is a new medicine.' Cognito is also targeting dementia.
Brent Vaughan, CEO of Cognito, stated, "Amyloid beta is produced in the brain, causing pathological phenomena, which in turn changes electrical signals. We confirmed that altering electrical activity regulates protein expression." Cognito revealed that gamma frequency neural changes reduced brain atrophy related to Alzheimer's progression by 65%.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.