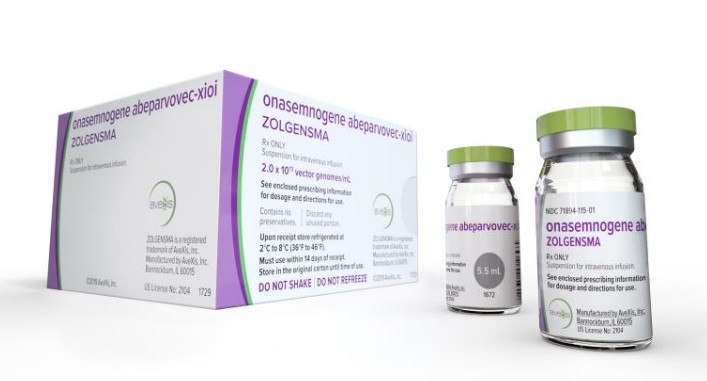
'One-shot treatment' Zolgensma likely to be covered by health insurance
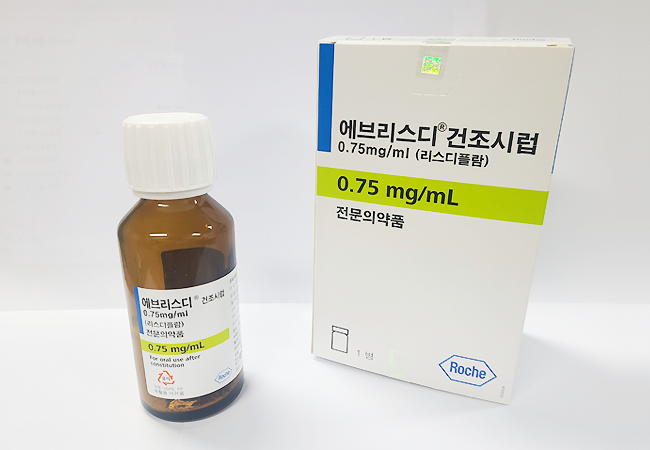
'Oral drug' Evrysdi also applying for coverage
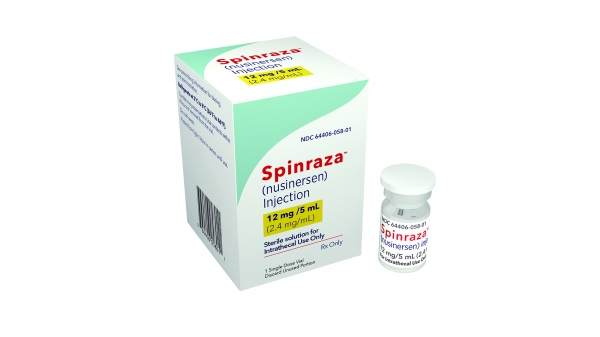
Competition emerging with existing treatment Spinraza
[Asia Economy Reporter Lee Chun-hee] As Novartis' spinal muscular atrophy (SMA) treatment 'Zolgensma' is likely to be covered by the National Health Insurance, fierce competition is expected as Roche's 'Evrysdi' is also aiming to enter the domestic market alongside the existing treatment Biogen's 'Spinraza'.
According to industry sources on the 6th, Zolgensma, which has attracted attention as a 'one-shot treatment', is likely to be covered as early as next month. Price negotiations are currently underway with the National Health Insurance Service.
SMA is a type of degenerative neurological disease in which spinal motor neurons disappear, causing muscle weakness or loss. It is known to occur in about 1 in 10,000 newborns. This means that 20 to 30 patients are diagnosed annually in South Korea alone. Although there was no treatment method until now, expectations for gene therapy have recently increased.
In particular, Zolgensma is known to produce effects close to a cure with a single administration. However, it is a high-priced drug costing 2.5 billion KRW in the U.S. and 1.9 billion KRW in Japan, placing a heavy burden on patients. Although the health insurance burden is significant, if coverage is granted, the patient's out-of-pocket cost is expected to be capped at around 6 million KRW.
The existing treatment used is Spinraza. Spinraza is also a high-priced drug costing 120 million KRW without insurance coverage, but after coverage, the patient's burden has been reduced to around 9 million KRW.
Evrysdi applied for insurance coverage last year, and related procedures are underway. It is highly likely to be covered within this year. The biggest advantage of Evrysdi is that it is an 'oral medication.' Compared to Spinraza and Zolgensma, which are injectable drugs, it offers greater convenience in administration. Especially for SMA patients who often have weakened spinal muscles accompanied by scoliosis, limiting spinal injection treatment, Evrysdi can be an alternative treatment option.
Its broad approval range is also an advantage. It has been approved domestically for patients aged two months and older, and recently in the U.S., the indication was expanded to include those under two months. In contrast, Spinraza's domestic coverage applies only to patients with symptom onset before age three, and Zolgensma is approved only for certain patient types. However, Evrysdi's treatment cost for the first year is also reported to be in the range of 300 million KRW annually.
If Evrysdi is covered following Zolgensma, intense market competition is expected. In the U.S., where all three drugs have entered, Spinraza's sales began to decline after Zolgensma's launch in 2019, and after Evrysdi entered the market in 2020, it has rapidly gained ground. However, due to the one-shot nature of Zolgensma, patient accumulation does not occur, and quarterly sales remain at around 100 to 150 million USD (approximately 130.7 billion to 196.1 billion KRW), whereas Evrysdi is quickly increasing its market share by leveraging the advantage of being an oral drug. Some analyses predict that by around 2026, Evrysdi will hold the highest market share in the U.S.
However, an industry official said, "Unlike the U.S., South Korea has a strong public insurance system," adding, "If Zolgensma crosses the threshold for coverage, it is highly likely to capture the highest market share by leveraging the characteristics of a one-shot treatment."
Biogen is also conducting various additional studies to maintain Spinraza's market share. Currently, clinical trials are underway for preemptive administration to infants who are asymptomatic but have confirmed SMA expression potential through genetic testing, as well as for switch therapy targeting patients with insufficient treatment effects after Spinraza treatment.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















