[Asia Economy Reporter Lee Gwan-joo] Bodytech Med announced on the 4th that it has been selected for a new support project under the Ministry of Trade, Industry and Energy's Bioindustry Technology Development Project.
This project, implemented to foster future new industries and enhance the competitiveness of key traditional industries through focused support for the development of core and foundational technologies in the bio sector, allocates a total support budget of 38.8 billion KRW, of which 24.75 billion KRW is designated for the Bioindustry Technology Development Project.
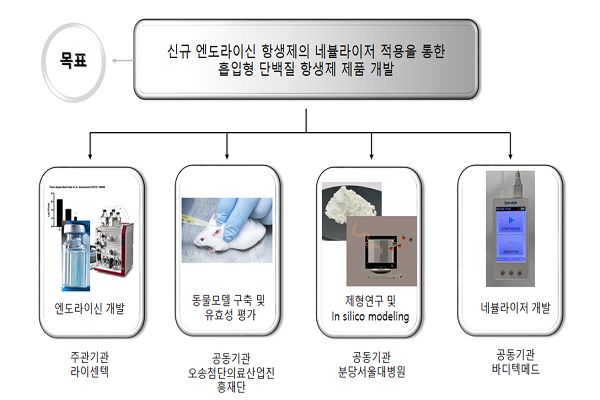
Bodytech Med was selected for the development of foundational technology for inhalable convergent products of biopharmaceuticals in the customized diagnostic and therapeutic field. Together with the lead institution LicenTec, and co-research institutions Bundang Seoul National University Hospital, University of Sydney, and Osong Advanced Medical Industry Promotion Foundation, the goal is to develop an inhalable protein antibiotic product by applying a new endolysin antibiotic to a nebulizer.
LicenTec is researching a new endolysin antibiotic with antimicrobial capabilities, Bundang Seoul National University Hospital and the University of Sydney are developing optimal formulations suitable for the nebulizer, and the Osong Advanced Medical Center is responsible for establishing animal models and conducting efficacy evaluations.
Bodytech Med aims to implement a commercial nebulizer that delivers the drug to the target in an optimal state by establishing parameters such as particle size of the drug, viscosity upon liquefaction, vibration speed and intensity of the ultrasonic vibrator, and appropriate usage temperature depending on the drug.
The nebulizer ‘SyncNeb’ being developed by Bodytech Med uses a vibrating mesh method rather than the jet method commonly used in intensive care units. It sprays the drug in uniform particles smaller than 5μm, allowing stable adhesion to the patient's lungs, resulting in a high drug delivery rate and low noise.
An inhalable therapeutic refers to both the device that delivers a fixed amount of drug directly to the lungs using an inhaler and the drug itself. It is essential for treating respiratory diseases such as asthma and COPD (chronic obstructive pulmonary disease).
Choi Eui-yeol, CEO of Bodytech Med, said, “Although there are no patent barriers for inhalable therapeutics, domestic companies have not entered this field due to technological barriers. Through participation in this national project, we will enhance our technological capabilities and break down the technological barriers of inhalable therapeutics with ‘SyncNeb,’ which improves the shortcomings of existing nebulizers, and lead the localization of medical devices.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















