HK Innoen 'K-CAB'
Only Domestic P-CAB Drug on Market
Outpatient Prescription Sales Surpass 100 Billion KRW
Daewoong Pharmaceutical 'Pexuclu'
Faces 'Drug Price' Challenges
Domestic Launch Scheduled for Second Half
Jeil Pharmaceutical Also Conducting Phase 3 Trials
Intense Competition to Capture Overseas Markets
[Asia Economy Reporter Lee Chun-hee] As the paradigm of the global gastroesophageal reflux disease (GERD) market, worth 42 trillion won, shifts, competition among domestic companies to dominate the market is intensifying.
GERD has traditionally been treated mainly with drugs based on the mechanism of proton pump inhibitors (PPIs). However, these drugs must be taken 30 minutes before meals and cause discomfort due to increased acid secretion at night. Side effects such as osteoporosis and stroke have also been controversial. Recently developed and launched potassium-competitive acid blockers (P-CABs), however, can be taken regardless of meal times and have improved nighttime heartburn symptoms.
Following HK Innoen, Daewoong Pharmaceutical Throws Down the Gauntlet
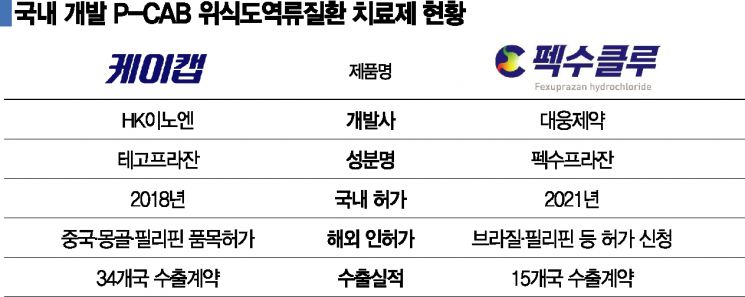
As of the 25th, HK Innoen’s ‘K-CAB’ (active ingredient: tegoprazan), the only P-CAB drug currently marketed domestically, has rapidly captured the market. Since its approval in 2018, K-CAB rose to become the number one drug in the peptic ulcer treatment market in 2020 and surpassed 100 billion won in outpatient prescription sales last year, quickly absorbing the domestic market.
Outpatient prescription sales of 100 billion won rank third among domestic new drugs, following Boryung Pharmaceutical’s ‘Kanab’ and LG Chem’s ‘Gemiglo,’ and were achieved within three years of launch. This is seen as breaking the harsh history of domestic new drugs. HK Innoen is not stopping there; it has launched an orally disintegrating tablet formulation and is expanding indications to include peptic ulcers and Helicobacter pylori eradication beyond GERD.
A notable challenger is Daewoong Pharmaceutical’s ‘Pexuclu’ (active ingredient: fexuprazan), which received domestic approval at the end of last year. However, although aiming for a domestic launch in the second half of the year, it is facing difficulties due to pricing issues. Recently, the Health Insurance Review & Assessment Service’s Drug Reimbursement Evaluation Committee granted conditional approval for Pexuclu under the condition of ‘acceptance below the evaluation price.’
Daewoong Pharmaceutical aimed for a higher price than K-CAB’s 1,300 won per tablet but was instead asked to accept a price around the weighted average of K-CAB and existing PPI treatments. Since PPI drug prices range from 700 to 1,100 won, it is likely that Pexuclu will receive a significantly lower price. Nevertheless, Daewoong Pharmaceutical is reportedly planning to launch the product despite potential profitability deterioration, as it has already set the second half of the year as the target.
Additionally, Jeil Pharmaceutical is developing ‘JP-1366’ through its subsidiary Onconic Therapeutics and is currently conducting Phase 3 clinical trials domestically.
Rapid Expansion into Overseas Markets
Competition for overseas expansion is also fierce. K-CAB, which was launched first, appears to be taking the lead in exports. Currently, K-CAB has export contracts with 34 countries, while Pexuclu has contracts with 15 countries. In terms of approvals, Pexuclu has no overseas approvals yet, whereas K-CAB has obtained sales approvals in China, the Philippines, Mongolia, and others. Notably, sales in China, the world’s largest peptic ulcer treatment market, began this month through local partner Luoxin. Daewoong Pharmaceutical is rapidly catching up by conducting Phase 3 clinical trials in China through technology exports.
As the global GERD treatment market, estimated at 42 trillion won, undergoes a paradigm shift to P-CABs, there is optimism that domestic companies could dominate the global market if they engage in healthy competition. This is partly because multinational pharmaceutical companies have yet to release P-CAB drugs except for Japan’s Takeda’s ‘Takecab’ (active ingredient: vonoprazan).
A top priority for this is entering the U.S. market through approval by the U.S. Food and Drug Administration (FDA). Although Takecab is approved only for Helicobacter pylori eradication, it recently succeeded in obtaining FDA approval. It also applied for FDA approval as a treatment for erosive esophagitis in March and aims to launch it next year.
Domestic pharmaceutical companies are also accelerating their U.S. market entry. Daewoong Pharmaceutical plans to start Phase 3 clinical trials in the U.S. within the year based on existing clinical data in collaboration with its U.S. partner Neurogastrics. HK Innoen is conducting Phase 1 clinical trials in the U.S. and has signed a technology export contract with Sevela for subsequent trials. Jeil Pharmaceutical is pushing forward with Phase 3 trials in Europe and has completed Phase 1 clinical trials for equivalence evaluation with Caucasians domestically, preparing for overseas expansion.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.


![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)













