Sanghyun Lee, CEO of Femtobiomed
CellShot Overcomes mRNA Technology Limitations
Cell Collection and Drug Manufacturing
Now Possible Within Hospitals
First Step Toward Commercialization Through Joint Research with University Hospitals
[Asia Economy Reporter Chunhee Lee] "Femto Biomed's Cellshot technology is the cheapest, safest, and easiest-to-distribute cell therapy manufacturing technology." (Sanghyun Lee, CEO of Femto Biomed)
One of the biggest topics in the bio industry recently is cell therapy. A representative example is Novartis' chimeric antigen receptor (CAR)-T cell therapy 'Kymriah,' known as the 'miraculous one-shot therapy' that can completely eliminate pediatric acute leukemia with just one dose. However, Kymriah sparked major controversy during its introduction in Korea due to its price of 360 million KRW. Despite its ultra-high cost, there was heated debate over whether it should be covered by health insurance given its efficacy, which is called a 'miracle.'
Sanghyun Lee, CEO of Femto Biomed, expressed confidence in an interview with Asia Economy on the 6th that the company's Cellshot technology will become an innovative technology that will change the future cell therapy market. He emphasized that using Cellshot lowers the price compared to existing cell therapy production technologies, improves safety, and lowers the technical barriers.
The current main production method for cell therapies like Kymriah is the 'viral vector' method. It requires a high level of Good Manufacturing Practice (GMP) certification, which demands significant costs to establish and maintain continuously. This is why not only existing Contract Development and Manufacturing Organizations (CDMOs) but also large corporations are rushing to acquire cell and gene therapy (CGT) CDMO companies. There are also concerns about side effects. Since the manipulated cells continue to reproduce and remain permanently in the body, there is an inherent risk of viral mutations.
To replace this, the recently emerging method is messenger RNA (mRNA). However, if mRNA is simply introduced into the body, our immune system recognizes it as a harmful substance and eliminates it, so a separate delivery material is required. For this reason, lipid nanoparticle (LNP) technology has attracted attention as a core technology for mRNA delivery. Another delivery technology gaining attention is electroporation (EP), but it also involves enormous costs. EP requires a special solution called a 'buffer,' which is produced by only a few companies holding patents, making it expensive. Additionally, there are concerns about yield reduction during the washing process to remove the solution from the cells.
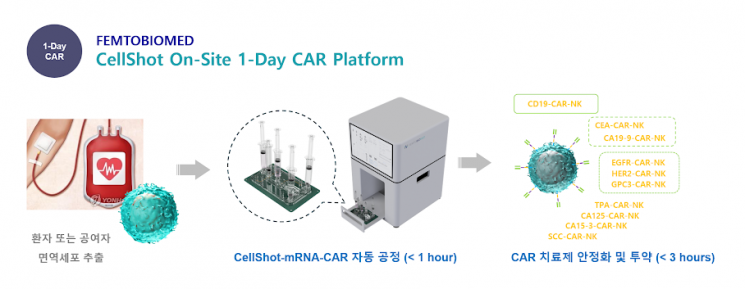
Femto Biomed's Cellshot overcomes these limitations with a separation delivery technology. It directly inserts mRNA into cells through a nano-injector made using femtosecond laser technology. Since the mRNA and cells, which are usually separated, only come into contact at the moment of insertion, all the drawbacks of EP are resolved. This process is carried out through a fully enclosed automated system. CEO Lee stated, "It is possible to manufacture with a machine about the size of a microwave oven, enabling all processes from cell collection to therapy manufacturing to be done within the hospital." He explained that since there is no human handling, GMP certification is much easier, and costs are significantly reduced because no patented buffer is used.
Last year, Femto Biomed achieved a milestone by developing mRNA delivery technology into NK cells with a processing speed of over 1 billion cells per hour. CEO Lee said, "It is a speed that can produce about 10 cell therapy doses per hour," adding, "This matches the technical level required in current production sites, and production can be increased anytime by improving processing speed and expanding machines."
The first step toward commercialization will also be taken this month. Joint research will begin with major university hospitals such as Yonsei Severance and Seoul St. Mary's Hospital. CEO Lee explained, "Using Cellshot technology, hospitals themselves can manufacture cell therapies as much as they want," adding, "This research aims to prove that in practice."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.


![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















