COVID-19 Vaccination for Unvaccinated Adults in Hangzhou
Sinopharm Vaccine Development Took 139 Days from Initiation to Clinical Trial Approval
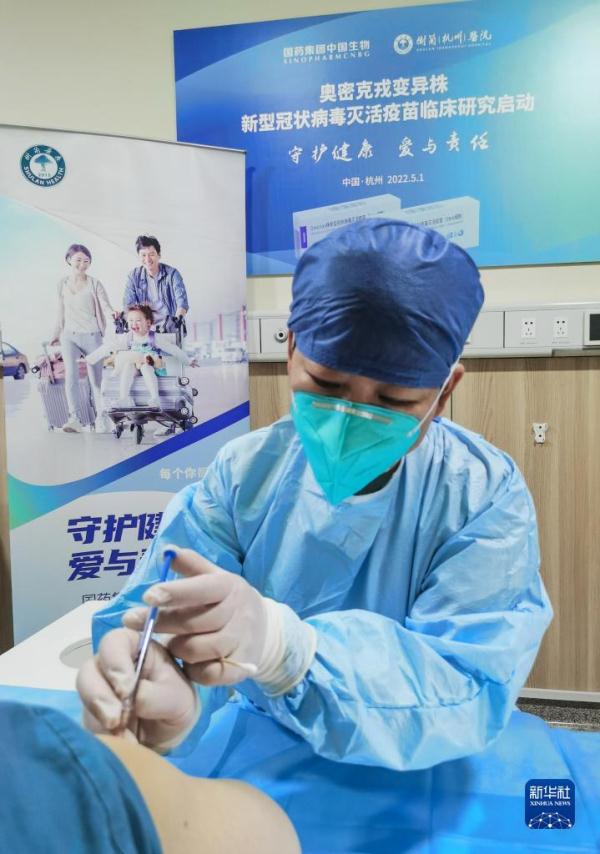
[Asia Economy Beijing=Special Correspondent Jo Young-shin] Chinese media reported that clinical trials for a COVID-19 Omicron variant-specific vaccine have begun in Hangzhou, Zhejiang Province.
On the 3rd, according to state-run Xinhua News Agency and other Chinese media, Sinopharm started clinical trials of the Omicron-specific vaccine for the general public at Suran Hospital in Hangzhou from the 1st.
Chinese media reported that vaccination was carried out after obtaining prior consent from clinical trial volunteers. Before vaccination, basic examinations such as blood sampling were conducted, the Chinese media added.
Chinese media explained that the Omicron variant-targeted vaccine clinical trial is the world's first, and this vaccine is an inactivated vaccine that can also generate antibodies against variants other than Omicron.
The clinical trial subjects are adults aged 18 and over who have not been vaccinated against COVID-19. Safety and immunogenicity will be evaluated after two doses.
The vaccine entering clinical trials this time was developed by the Chinese pharmaceutical company Sinopharm, and Chinese health authorities approved the clinical trial on the 26th of last month. Sinopharm secured the Omicron virus at the University of Hong Kong on December 9 last year and has been developing the vaccine since then. The period from vaccine development to clinical trials is less than five months. Chinese media did not disclose the exact number of participants in phase 1 of this clinical trial.
Li Lanjuan, an academician of the Chinese Academy of Engineering, expressed expectations, saying, "Existing COVID-19 vaccines are effective against the Omicron variant, but if the newly developed vaccine succeeds in clinical trials, its effectiveness will be even better." Li also explained that additional clinical trials will be conducted targeting people who have received existing COVID-19 vaccines.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















