[Asia Economy Reporter Lee Chun-hee] SK Bioscience's domestically developed first COVID-19 vaccine, 'SKYCovione Multi,' has entered the domestic approval process.
On the 29th, SK Bioscience announced that it had completed the application for product approval to the Ministry of Food and Drug Safety (MFDS) following the successful completion of the Phase 3 clinical trial of its self-developed COVID-19 vaccine candidate ‘GBP510.’ The MFDS also announced on the same day that it had begun the review process for the product.
The official product name of GBP510 was decided as ‘SKYCovione Multi.’ The company explained, "It embodies the ambition to become the only (one) vaccine leading the global vaccine market as South Korea's first COVID-19 vaccine."
SKYCovione is a synthetic antigen-based COVID-19 vaccine developed through collaboration between SK Bioscience and global organizations and companies. From the early development stage, it received development funding from the Bill & Melinda Gates Foundation (BMGF) and the Coalition for Epidemic Preparedness Innovations (CEPI). It was co-developed by the Institute for Protein Design (IPD) at the University of Washington School of Pharmacy and SK Bioscience. Additionally, GlaxoSmithKline (GSK)'s adjuvant 'AS03' was applied to enhance immune response and induce a high level of neutralizing antibodies.
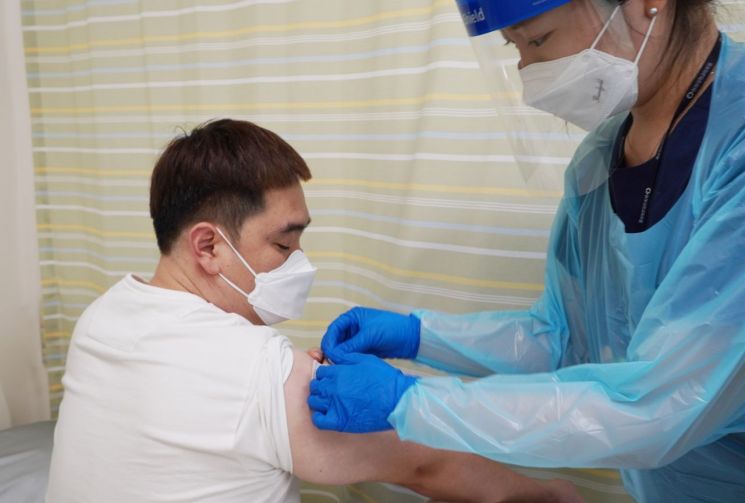 In August, subject administration for the Phase 3 clinical trial of SK Bioscience's COVID-19 vaccine, Skycovione Multi, was conducted at Dong-A University Hospital in Busan. (Photo by SK Bioscience)
In August, subject administration for the Phase 3 clinical trial of SK Bioscience's COVID-19 vaccine, Skycovione Multi, was conducted at Dong-A University Hospital in Busan. (Photo by SK Bioscience)
Previously, SK Bioscience demonstrated the superiority of SKYCovione in immunogenicity and safety compared to the control vaccine, AstraZeneca, in a Phase 3 clinical trial involving 4,037 adults aged 18 and over across five countries, including South Korea and overseas.
According to the clinical results, after two doses of SKYCovione, the neutralizing antibodies that neutralize and prevent COVID-19 infection were formed at a level 2.93 times higher than the control vaccine, showing a significantly superior level. The ‘seroconversion rate,’ which refers to the proportion of subjects whose neutralizing antibody levels increased more than fourfold after vaccination, was also 98%, more than 10 percentage points higher than the control vaccine’s 87%, showing a statistically significant difference. Notably, in the elderly group aged 65 and over, the seroconversion rate in the SKYCovione group exceeded 95%, showing a large difference compared to the control vaccine’s 79%, indicating that SKYCovione will be effectively used to protect the elderly in endemic (periodic outbreak) situations. The cellular immune response, which plays an important role in reducing the severity of COVID-19 infection, was also confirmed to be at least equivalent to that of the control vaccine.
In terms of safety, SKYCovione showed a similar adverse reaction rate compared to the control vaccine. No particular safety issues were reported during the clinical trial period. The synthetic antigen-based SKYCovione is expected to provide a new vaccine option for those who have refused vaccination due to safety concerns about existing vaccines.
It is also efficient in distribution and storage. Unlike existing messenger RNA (mRNA) vaccines that require ultra-low temperature storage, SKYCovione can be distributed refrigerated at 2?8 degrees Celsius and stored long-term. This is expected to play a role in increasing vaccination rates in underdeveloped countries that have struggled to supply vaccines due to the lack of expensive ultra-low temperature facilities.
The product approval for SKYCovione is proceeding through an expedited approval process for full product authorization, not emergency use authorization. The MFDS plans to decide on approval after triple advisory reviews, including the verification advisory group, the Central Pharmaceutical Review Committee, and the final inspection committee, similar to existing COVID-19 vaccines. The MFDS explained, "If the submitted data are appropriate, approval could be possible as early as June."
Considering the overall procedures for actual vaccination thereafter, commercialization is expected to take place in the second half of the year. Earlier, SK Bioscience signed a domestic supply contract worth 200 billion KRW to supply 10 million doses of SKYCovione to the Korea Disease Control and Prevention Agency in March.
After obtaining domestic approval, SKYCovione is scheduled to be supplied worldwide through the global vaccine procurement project ‘COVAX Facility.’ SK Bioscience’s strategy is to target the global market by obtaining emergency use listing (EUL) from the World Health Organization (WHO) and emergency use authorizations from overseas countries such as Europe.
 President-elect Yoon Suk-yeol is touring the laboratory during his visit to SK Bioscience in Bundang-gu, Seongnam-si, Gyeonggi Province on the morning of the 25th. [Provided by the Office of the President-elect Spokesperson]
President-elect Yoon Suk-yeol is touring the laboratory during his visit to SK Bioscience in Bundang-gu, Seongnam-si, Gyeonggi Province on the morning of the 25th. [Provided by the Office of the President-elect Spokesperson]
Roger Connor, Head of GSK Global Vaccine Business, said, “As repeated variants emerge and COVID-19 is predicted to become endemic, demand for vaccines that are easy to distribute is expected to continuously increase. SKYCovione not only secures excellent immunogenicity and safety but also allows for ambient temperature distribution, so I am confident it will play a very important role in epidemic control in the endemic era.”
SK Bioscience President Ahn Jae-yong also said, “At this moment when countries around the world are devising strategies to respond to the COVID-19 endemic era, it is a moving moment that South Korea’s first COVID-19 vaccine has reached the final stage for release. We will continue to cooperate with global organizations and companies and do our best to become an innovative bio company competing globally.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















