Professor Hyungyu Park's Team at KAIST
[Asia Economy Reporter Kim Bong-su] A technology has been developed that uses gene scissors to detect causative agents of AIDS (Acquired Immunodeficiency Syndrome) and hepatitis B.
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 14th that a research team led by Professor Park Hyun-gyu of the Department of Biological and Chemical Engineering developed a new technology that sensitively detects RNA-degrading enzymes by utilizing the collateral cleavage activity of the CRISPR-Cas12a system.
The CRISPR-Cas system is an adaptive immune system evolved by bacteria to protect themselves from viral infections. It consists of guide RNA containing foreign genetic information and Cas proteins that directly cleave nucleic acids. It became widely known after Professor Jennifer Doudna's team developed the CRISPR-Cas9 gene editing system and won the Nobel Prize in Chemistry in 2020. Due to its high target specificity and rapid kinetics, it has recently been extensively applied beyond genome editing to biomolecule detection and molecular diagnostics.
In addition to Cas9, various Cas proteins such as Cas12 and Cas13 have been discovered and utilized. Cas12a recognizes and cleaves target DNA sequences and additionally exhibits collateral cleavage activity that randomly cuts nearby non-target single-stranded DNA. This property is actively used in the field of molecular diagnostics.
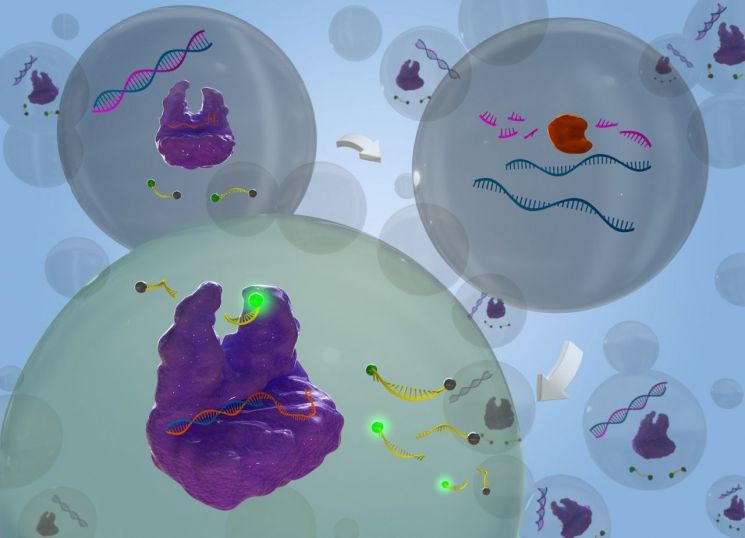
Ribonuclease H, a type of RNA-degrading enzyme, is an essential domain in reverse transcriptases of retroviruses including human immunodeficiency virus (HIV-1), which causes AIDS, and hepatitis B virus. It is involved in the proliferation of retroviruses. Therefore, ribonuclease H is known as an important target for antiviral drug development. Generally, methods such as electrophoresis or high-performance liquid chromatography are used to detect ribonuclease H activity, but these techniques have drawbacks such as low specificity and sensitivity, complex detection processes, and long detection times.
To overcome these limitations of current technologies, the research team utilized the CRISPR-Cas12a system to greatly enhance detection sensitivity and succeeded in detecting ribonuclease H with the highest sensitivity reported to date (detection limit: 0.24 U/L) within one hour.
The team designed a short DNA/RNA chimera complex as a substrate for ribonuclease H so that under ribonuclease H activity, activator DNA (Activator DNA, AD) would be released. When the Cas12a/crRNA complex recognizes the released activator DNA, Cas12a's collateral cleavage activity is activated, cleaving nearby reporter DNA to generate a fluorescent signal, enabling highly sensitive and accurate detection of target gene mutations. Using this technology, the team successfully detected ribonuclease H activity in cancer cells.
Considering that ribonuclease H is involved in the proliferation of human immunodeficiency virus, this research achievement is also expected to contribute to the development of AIDS treatments.
Professor Park Hyun-gyu explained the significance of the research, stating, “This technology utilizes the collateral cleavage activity of the CRISPR-Cas12a system to highly sensitively detect ribonuclease H, which can be used to identify targets for antiviral drug development.”
The research results were selected as the back cover paper of issue 16 in 2022 of the international journal Chemical Communications, published by the Royal Society of Chemistry in the UK, on the 24th of last month.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.