Recruitment of 4,037 Participants Completed
Approval Targeted for First Half of This Year
Initial Response... Urgent Need for Further Development
[Asia Economy Reporters Chunhee Lee and Daehyun Kim] SK Bioscience’s ‘GBP510,’ the first domestically developed COVID-19 vaccine, is smoothly progressing toward the final stage of development. Although questions have been raised about its efficacy amid the rapid spread of the Omicron variant, there are voices calling for faster development since once the vaccine targeting the initial virus is completed, it will be possible to respond to variants thereafter.
SK Bioscience Aims for Approval in the First Half of the Year
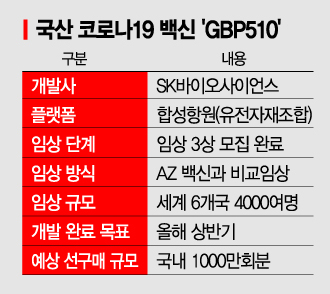
On the morning of the 18th, SK Bioscience CEO Jaeyong Ahn presented the progress of the Phase 3 clinical trial of the COVID-19 vaccine at the ‘Government-wide COVID-19 Vaccine and Therapeutics Clinical Trial Support Task Force (TF)’ meeting. SK Bioscience is conducting a Phase 3 clinical trial comparing its vaccine with the AstraZeneca (AZ) vaccine, targeting a total of 3,990 participants. Since the first dose on August 30 last year, recruitment of 4,037 participants has been completed across six countries: South Korea, Thailand, the Philippines, Vietnam, Ukraine, and New Zealand. A SK Bioscience official explained, “We are coordinating dosing schedules with participants and will complete dosing as quickly as possible.” The goal is domestic approval within the first half of this year. The government has already allocated a budget this year to pre-purchase 10 million doses of a domestic vaccine, with GBP510 being the most likely candidate.
Evaluation of vaccine efficacy (neutralizing antibody analysis) to confirm actual effectiveness is also underway. The Korea Disease Control and Prevention Agency’s National Institute of Health is conducting efficacy evaluation on Phase 3 clinical samples in cooperation with the International Vaccine Institute (IVI). As of the day before, 2,163 samples have been received, and analysis of 1,764 samples has been completed. Approximately 4,400 sample analyses are required for expedited approval application to the Ministry of Food and Drug Safety. Samples from 570 domestic participants have been received, and SK Bioscience plans to quickly import samples from 3,467 overseas participants to accelerate analysis. In earlier Phase 1 and 2 trials, over 99% of the group administered with the vaccine plus an adjuvant showed formation of neutralizing antibodies that neutralize the COVID-19 virus.
Since vaccination rates for those aged 12 and older in South Korea have already reached 94.5% for the first dose and 92.6% for the second dose, effectively completing most vaccinations, research on heterologous (mix-and-match) and booster vaccinations is also underway. Currently, under the supervision of the Korea Disease Control and Prevention Agency, a researcher-led clinical trial involving 550 participants is being conducted on heterologous and booster doses of GBP510. Previously, the Novavax vaccine was approved only for the primary 1st and 2nd doses due to insufficient additional clinical data.
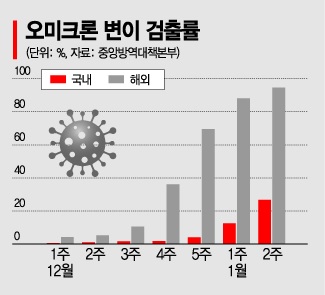
However, the efficacy against rapidly spreading variants such as Omicron remains uncertain, posing a challenge. This is because the clinical trials of GBP510 are conducted against the original ‘Wuhan strain.’ The health authorities predict that the Omicron variant will become dominant, surpassing a 50% detection rate by this weekend. According to the Central Disease Control Headquarters, the domestic detection rate of the Omicron variant has rapidly increased over the past three weeks from 4.0% to 12.5% and then to 26.7%. Notably, last week’s detection rate among imported cases nearly reached 94.7%. As of the 15th, the number of infected individuals reached a total of 5,030, with 2,391 domestic and 2,639 overseas cases.
"Even If Omicron Can't Be Controlled Immediately, Continued Investment Is Necessary"
Although the Omicron variant is known to have lower severity and fatality rates compared to Delta, its transmissibility is 2 to 3 times higher, raising concerns that its actual risk may be greater than Delta. Especially, many mutations in the spike protein region, where the virus binds to host cells, have raised fears that the infection prevention effect of existing vaccines may decrease. In South Korea, as the third dose vaccination campaign intensifies and quarantine measures strengthen, the number of new confirmed cases, which had been declining, is now increasing again alongside the rising detection rate of the Omicron variant. Health authorities and experts worry that depending on the level of social distancing, daily new cases could reach 20,000 by the end of next month and over 30,000 by the end of March, potentially overwhelming quarantine and medical response capacities.
SK Bioscience is also reviewing whether neutralizing antibody formation can be confirmed at the preclinical stage against variants such as Delta and Omicron. Based on the GBP510 platform, the company is developing a vaccine targeting the entire Sarbecovirus genus. Sarbecoviruses are a group of coronaviruses that include variants related to COVID-19 and Severe Acute Respiratory Syndrome (SARS) viruses.
An industry insider said, “Some pharmaceutical companies plan to skip vaccine development against the Wuhan strain and directly develop variant-specific vaccines, but considering the difficulty of development, this may be challenging,” adding, “Developing a basic vaccine first and then developing variant vaccines is more efficient in terms of feasibility and speed than developing an Omicron-specific vaccine.”
Professor Jaehun Jung of the Department of Preventive Medicine at Gachon University College of Medicine also said, “Even if vaccine development is completed in the first half of the year, it will not immediately be effective against the recent outbreak,” but emphasized, “Domestic vaccines are more about long-term investment and response.” He added, “The domestic vaccine development process could lead to the development of an Omicron-specific vaccine. We need to approach this from a long-term perspective to prepare for another possible pandemic in the future.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














