Deployment at Vaccination Sites as Early as Next Month
Developed into 'Single-Use Syringe' Prefilled Syringe through Additional R&D by SK Bioscience
Traditional Vaccine Development Method Minimizes Side Effect Concerns
90% Prevention Effectiveness · 100% Severe Case Prevention
Cross Vaccination and Third Dose to Be Reviewed Later
Effectiveness Against Omicron Variant Unknown
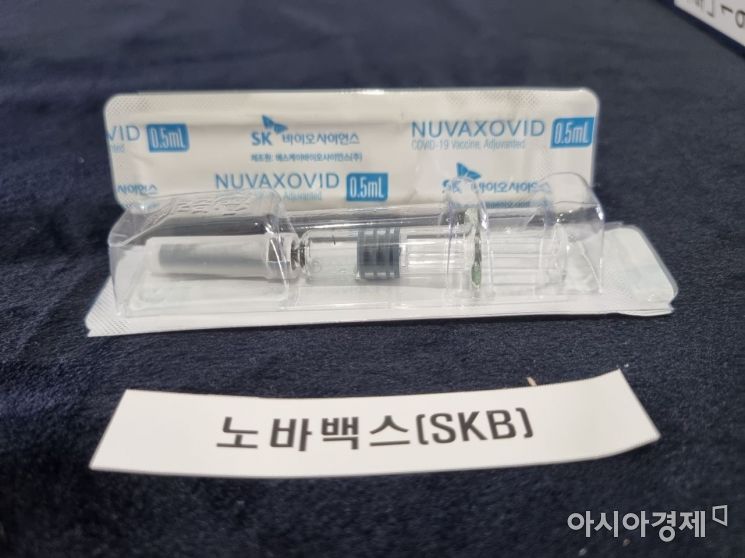 SK Bioscience's contract development and manufacturing organization (CDMO) product, Novavax COVID-19 vaccine 'Nuvaxovid Prefilled Syringe'. /Photo by Lee Chunhee
SK Bioscience's contract development and manufacturing organization (CDMO) product, Novavax COVID-19 vaccine 'Nuvaxovid Prefilled Syringe'. /Photo by Lee Chunhee
[Asia Economy Reporter Lee Chun-hee] The COVID-19 vaccine 'Nuvaxovid Prefilled Syringe,' developed by Novavax and contract-manufactured (CMO) by SK Bioscience, has received product approval from the Ministry of Food and Drug Safety (MFDS). Developed using the traditional vaccine manufacturing method of recombinant antigen (genetic recombination), it is expected to have fewer side effects compared to existing vaccines, making it a promising alternative for unvaccinated individuals who have delayed vaccination due to concerns about side effects.
On the afternoon of the 12th, Kim Gang-lip, head of the MFDS, stated, "Today (the 12th), the MFDS held a final review committee meeting and decided to grant product approval for the COVID-19 vaccine Nuvaxovid Prefilled Syringe, for which SK Bioscience applied for manufacturing and marketing authorization, on the condition that the final clinical trial report and other documents are submitted." Following manufacturing and national batch release approval, the vaccine is expected to be used for vaccinations in the field around next month.
Previously, the MFDS received advisory opinions from the verification advisory group on the 29th of last month and from the Central Pharmaceutical Affairs Deliberation Committee on the 6th of this month. The final product approval decision was made at today's meeting, the last stage of the approval review process. The meeting held this morning was attended by three external experts, including Oh Il-hwan, chairman of the Central Pharmaceutical Affairs Committee, and five internal members including the MFDS chief.
Developed Using Traditional Vaccine Method… SK Bioscience Enhances Safety by Developing Single-Dose Syringe
Nuvaxovid Prefilled Syringe is a recombinant antigen vaccine developed by Novavax in the United States and manufactured by SK Bioscience from raw material to finished product. It uses recombinant technology to directly inject antigen proteins that induce antibody production within the body. This method has been traditionally used in various vaccines such as influenza, hepatitis B, and cervical cancer.
Our government signed a contract last year to supply 40 million doses of the Novavax COVID-19 vaccine, but since domestic approval was not granted, all of this quantity has been carried over to this year. SK Bioscience holds all domestic production and commercialization rights. SK Bioscience signed a contract for contract development and manufacturing (CDMO) with Novavax in August 2020 and subsequently signed a technology transfer agreement in February last year to produce both raw materials and finished pharmaceuticals.
 Novavax COVID-19 vaccine 'Nuvaxovid Prefilled Syringe' is being produced at the L House factory of Andong SK Bioscience. (Photo by SK Bioscience)
Novavax COVID-19 vaccine 'Nuvaxovid Prefilled Syringe' is being produced at the L House factory of Andong SK Bioscience. (Photo by SK Bioscience)
Notably, while Novavax vaccines are mostly distributed overseas in vials containing 10 doses, SK Bioscience's Nuvaxovid Prefilled Syringe has been improved through additional R&D to a single-dose syringe form containing one dose per vaccine. This eliminates the need for dilution or aliquoting procedures, freeing it from concerns about contamination during these processes and reducing the workload for medical personnel. This is unique among vaccines currently approved in Korea, including AstraZeneca (AZ), Pfizer, Moderna, Janssen, and Novavax vaccines. All other vaccines are packaged in multi-dose vials and administered by aliquoting.
However, due to this development method, the vaccine review period, initially announced by the MFDS as '40 days,' took 58 days for Nuvaxovid Prefilled Syringe, from the application on November 15 last year to today. Director Kim explained the delay, saying, "Compared to previously produced vial products, more thorough additional reviews of quality safety and consistency were necessary."
The MFDS determined the efficacy and effectiveness of the Novavax vaccine as prevention of COVID-19 in individuals aged 18 and older, with a dosage regimen of 0.5 ml administered twice at 21-day intervals. Storage conditions were set at refrigerated temperatures of 2 to 8 degrees Celsius for five months.
The Novavax vaccine supplied domestically will initially be used for unvaccinated individuals who have not yet received basic vaccination. Since concerns about side effects are low, it is expected to improve vaccination rates by enabling basic vaccination for those who have refused vaccination due to side effect concerns related to viral vector (AstraZeneca, Janssen) or messenger RNA (mRNA, Pfizer, Moderna) vaccines.
The MFDS stated that additional review is needed for the third dose. Director Kim said about the Novavax vaccine, "It has been approved for basic vaccination," and added, "We understand that the developer is currently conducting separate clinical trials related to this, and if a request for approval change is submitted, the review for approval change will proceed based on the submitted data."
However, Pfizer, Moderna, and Janssen vaccines currently used for the third dose have been administered without undergoing approval change procedures. Regarding this, Director Kim explained, "Even if approval has not been changed, additional doses can be administered under the 'off-label use' of drugs for products with sufficient clinical experience and judgment accumulated in the field." Unlike other vaccines that have already undergone clinical trials for the third dose, Novavax has not conducted clinical trials related to the third dose, so it cannot be reviewed at this time. SK Bioscience explained, "Novavax disclosed data showing that antibody titers increased 4.6 times after two doses of Nuvaxovid followed by one booster shot six months later."
90% Prevention Effectiveness, 100% Severe Disease Prevention… Omicron Prevention Effect Unknown
The final review committee used phase 3 clinical trial data conducted in the UK (15,139 participants) and the US (29,582 participants) as the main data to evaluate the safety and efficacy of the Novavax vaccine.
Regarding safety, the committee judged that most reported adverse events were expected and related to vaccine administration and were generally mild. Common adverse events included tenderness, pain at the injection site, erythema, and swelling, with symptoms mostly mild to moderate and resolving within 1 to 3 days.
Furthermore, 'serious adverse events' were reported in 0.6% (44 people) of the vaccine group and 0.6% (44 people) of the control group in the UK trial with 15,139 participants, and in 0.9% (169 people) of the vaccine group and 1.0% (94 people) of the control group in the US trial with 29,582 participants. Among these, 'serious adverse drug reactions' possibly related to vaccine administration included one case of myocarditis in the UK and four cases in the US: angioedema, central nervous system inflammation/facial nerve palsy/peripheral neuropathy, Graves' disease/hyperthyroidism, and thrombocytopenia. All had recovered or were recovering at the time of clinical trial data submission.
The committee decided on post-approval safety measures to monitor myocarditis and other safety concerns preventively through a risk management plan and to collect and evaluate adverse events occurring during ongoing clinical trials and post-approval use.
The final review committee also recognized the vaccine's preventive effectiveness. In the UK trial, seven days after the second dose, 10 people in the vaccine group and 96 in the control group were confirmed COVID-19 positive, showing about 89.7% effectiveness. In the US trial, 14 in the vaccine group and 63 in the control group were confirmed positive, showing about 90.4% effectiveness. Notably, no severe COVID-19 cases occurred in the vaccine group, while five in the UK and four in the US control groups developed severe disease.
Neutralizing antibody evaluation, an indirect indicator of vaccine efficacy, showed that antibody titers increased more than fourfold in all recipients two weeks after the second dose compared to before vaccination.
However, the MFDS took a cautious stance on the vaccine's effectiveness against the recently spreading Omicron variant. Director Kim said, "The clinical trial results reviewed were conducted before the Omicron variant emerged," adding, "Additional data must be submitted for evaluation, and we will inform the public of the results." SK Bioscience explained, "After two doses of Nuvaxovid and a booster shot six months later, antibody titers responding to the Omicron variant were 9.3 times higher compared to after two doses."
Another weakness of the Novavax vaccine is that overseas approvals have not been fully completed. Although the vaccine has received emergency use authorization from the World Health Organization (WHO) and the European Medicines Agency (EMA), it has yet to apply for approval from the U.S. Food and Drug Administration (FDA), contrary to the initial plan to obtain approval within last year.
 Kim Gang-rip, Commissioner of the Ministry of Food and Drug Safety, held a briefing on the afternoon of the 12th at the MFDS conference room in Cheongju, Chungbuk, announcing the approval of the Novavax COVID-19 vaccine.
Kim Gang-rip, Commissioner of the Ministry of Food and Drug Safety, held a briefing on the afternoon of the 12th at the MFDS conference room in Cheongju, Chungbuk, announcing the approval of the Novavax COVID-19 vaccine. [Image source=Yonhap News]
Director Kim evaluated Nuvaxovid Prefilled Syringe, saying, "It is meaningful that it is manufactured using the recombinant genetic method, which the public has sufficient vaccination experience with, that it is convenient to administer as a single-dose syringe, that it can be stored refrigerated making storage and transport easier, and that it expands the types of vaccines available in medical settings."
SK Bioscience President Ahn Jae-yong said, "Nuvaxovid, verified for efficacy and safety through the MFDS's thorough review, will be a new key to controlling the current pandemic situation," adding, "As it is a vaccine produced with SK's proven technology, we will supply sufficient quantities domestically through consultation with the government."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















