Initial shipment of Paxlovid arrives tomorrow
Actual prescriptions start from the 14th
Additional 10,000 doses by the end of this month
Novavax vaccine final review results announced this afternoon
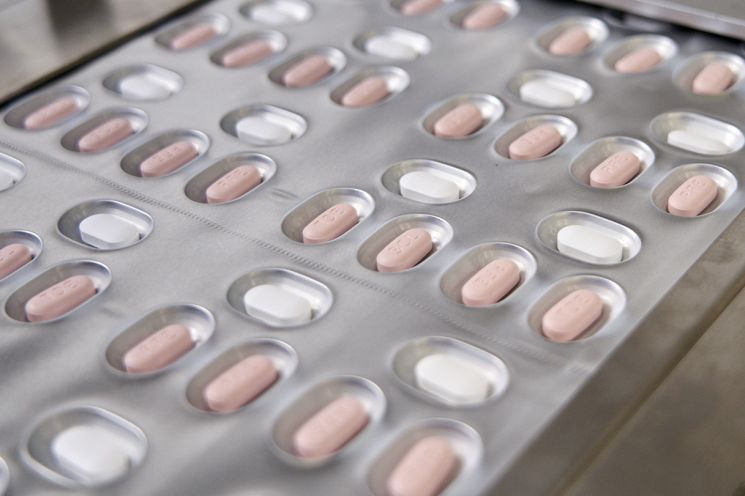
[Asia Economy Reporter Lee Chun-hee] On the 13th, 21,000 doses of Pfizer's oral COVID-19 treatment, Paxlovid, will arrive in South Korea. The government plans to import an additional 10,000 doses by the end of January, sequentially supplying a total of 31,000 doses this month.
The approval decision for the Novavax vaccine, developed using a recombinant antigen method and expected to have fewer side effects compared to existing vaccines, will also be made on the afternoon of the 12th. If the Novavax vaccine receives approval from the Ministry of Food and Drug Safety, it will become the fifth vaccine used domestically after AstraZeneca, Pfizer, Moderna, and Janssen.
A total of 31,000 doses of Paxlovid to be introduced by the end of this month
Jeon Hae-cheol, the 2nd Deputy Head of the Central Disaster and Safety Countermeasures Headquarters (Minister of the Ministry of the Interior and Safety), stated on the 12th, "Tomorrow (the 13th), 21,000 doses of the oral treatment produced by Pfizer are scheduled to arrive in the country," adding, "Priority will be given to administering the drug to elderly patients aged 65 and over and immunocompromised individuals who show mild to moderate symptoms within five days of symptom onset among those receiving home treatment or admitted to residential treatment centers."
Currently, the government has secured a total of 1,004,000 doses of oral treatments, combining 762,000 doses of Paxlovid and 242,000 doses of Merck (MSD)'s Molnupiravir. Among these, the initial batch of Paxlovid will arrive domestically for the first time on the afternoon of the 13th. An additional 10,000 doses will be imported by the end of this month, bringing the total supply to 31,000 doses.
Actual prescriptions will begin on the 14th. Priority for administration will be given to home-treated patients or those admitted to residential treatment centers who are aged 65 or older or immunocompromised, showing mild to moderate symptoms within five days of symptom onset and at high risk of progressing to severe illness. Considering the timing, those who developed symptoms from the 10th onward will be eligible to take the medication. Asymptomatic individuals are excluded from prescription eligibility.
Prescriptions will be carried out at 91 residential treatment centers nationwide and 281 designated pharmacies. Home-treated patients will receive the medication through non-face-to-face consultations followed by delivery via local governments or designated pharmacies. Guardians or others can visit the designated pharmacies to collect the medication, but in unavoidable cases, the health centers will deliver the medication directly. Patients admitted to residential treatment centers will receive medication through dedicated medical staff.
The government plans to expedite the confirmation of eligible patients by shortening schedules such as basic epidemiological investigations and initial patient classification, as oral treatments need to be taken within five days of symptom onset. The goal is to confirm eligible patients within 1 to 1.5 days after symptom onset.
Authorities will initially limit prescription eligibility due to the limited supply but plan to gradually expand the target group. Deputy Head Jeon said, "As the supply of treatments increases in the first quarter, we will expand the scope of administration focusing on those aged 60 and above with underlying conditions who are at high risk of severe progression." Currently, the emergency use authorization for Paxlovid covers adults with mild to moderate symptoms at high risk of severe progression and children aged 12 and older weighing 40 kg or more.
Since many medications should not be taken concurrently with Paxlovid, systematic management of administration will be conducted through related systems. To prevent co-administration with such drugs, the Drug Utilization Review (DUR) service, which allows checking prescription history, will be used to decide on administration and verify overlapping prescription histories.
Ritonavir, a component of Paxlovid, inhibits the enzyme 'CYP3A' responsible for metabolizing certain drugs, so Paxlovid should not be taken if the patient is on medications affected by this metabolic process. Medications that cannot be taken with Paxlovid due to potentially life-threatening interactions include: ▲Painkillers (pethidine, piroxicam, propoxyphene) ▲Anti-anginal agents (ranolazine) ▲Antiarrhythmics (dronedarone) ▲Anti-gout agents (colchicine) ▲Antipsychotics (lurasidone, pimozide, clozapine) ▲Cholesterol-lowering drugs (lovastatin, simvastatin) ▲Pulmonary arterial hypertension (PAH) treatments (sildenafil) ▲Sedatives and hypnotics (triazolam, oral midazolam).
Additionally, six substances including ▲Tuberculosis medication (rifampicin) ▲Anticancer agent (apalutamide) ▲Antidepressant (St. John's Wort) ▲Antiepileptics (carbamazepine, phenobarbital, phenytoin) may reduce the effectiveness of Paxlovid. Patients taking these drugs must discontinue them and wait a certain period before taking Paxlovid.
Novavax, the fifth vaccine approved domestically
On the same day, the Ministry of Food and Drug Safety is scheduled to announce the results of the final review committee in the afternoon to decide on the approval of the Novavax vaccine. The ministry conducts a three-tier advisory process before approving COVID-19 vaccines and treatments, including the Verification Advisory Group, the Central Pharmaceutical Review Committee, and the Final Review Committee. Since the vaccine has reportedly passed earlier review stages smoothly, it is highly likely to pass the final review as well.
If approved, it will become the fifth vaccine authorized domestically after AstraZeneca, Pfizer, Moderna, and Janssen. The Novavax vaccine is developed using a recombinant antigen (protein subunit) method, unlike the viral vector (AstraZeneca, Janssen) or messenger RNA (mRNA, Pfizer, Moderna) platforms used in existing COVID-19 vaccines, generating high expectations.
While research on viral vector and mRNA vaccines has been ongoing for a long time but only recently commercialized, the recombinant antigen method has traditionally been widely used in vaccines for influenza, hepatitis B, cervical cancer, and others. It is expected to have a low risk of side effects from vaccination. This allows basic vaccination for unvaccinated individuals who have refused vaccination due to concerns about side effects from viral vector or mRNA vaccines, potentially improving vaccination rates.
The government signed a contract last year to receive 40 million doses of the Novavax COVID-19 vaccine, but since domestic approval has not yet been granted, the entire quantity has been deferred to this year. SK Bioscience holds all domestic production and commercialization rights. SK Bioscience signed a contract for contract development and manufacturing organization (CDMO) with Novavax in August 2020 and later, in February last year, signed a technology transfer agreement to produce both bulk and finished pharmaceutical products.
However, the fact that overseas approval has not been fully granted remains a variable. The Novavax vaccine has received emergency use authorization from the World Health Organization (WHO) and the European Medicines Agency (EMA), but unlike the initial plan to obtain approval from the U.S. Food and Drug Administration (FDA) by the end of last year, the application has not yet been submitted.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.



![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















