Research Team of Lee Hyoyoung at the Institute for Basic Science
[Asia Economy Reporter Kim Bong-su] A technology has been developed that produces hydrogen by decomposing abundant urea in wastewater instead of water, significantly reducing the amount of electricity input and controlling water pollution.
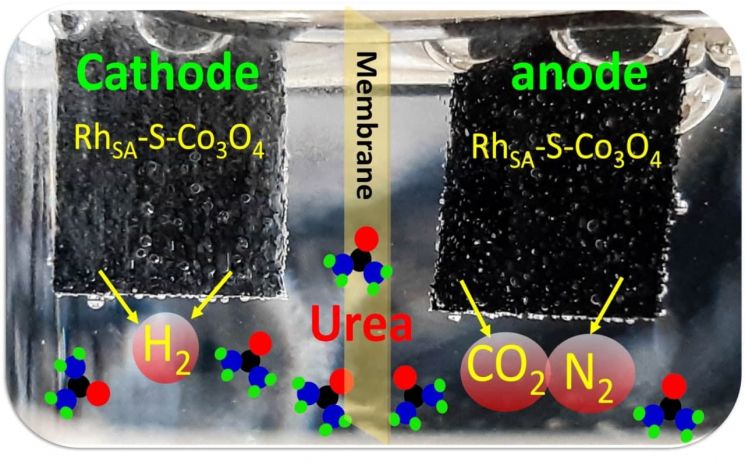
The Institute for Basic Science (IBS) announced on the 14th that the research team led by Deputy Director Lee Hyo-young of the Center for Nanostructured Physics developed a high-performance catalyst that accelerates the urea oxidation reaction by loading a large amount of single metal atoms. It is evaluated as presenting a new direction for green hydrogen production by increasing hydrogen production at a lower power than water splitting methods. Additionally, producing hydrogen using urea-rich wastewater is expected to alleviate water pollution problems as well as contribute to green hydrogen production.
Precious metal-based platinum (Pt) and rhodium (Rh) metal catalysts are used to accelerate the urea oxidation reaction, but they are very expensive and have poor catalyst performance and stability during long-term operation. Recently, single-atom catalysts, which show superior activity compared to nanomaterial-based catalysts, have attracted attention; however, they tend to migrate and aggregate easily, making it difficult to load a large amount of single atoms.
The research team developed a new surface deformation strategy to achieve ultra-high loading and stabilization of single metal atoms. Using a liquid nitrogen quenching method, they created deformations on the surface of cobalt oxide (Co3O4). The rapidly cooled oxide support surface undergoes deformation due to thermal contraction, and on this surface, they stabilized twice the amount of rhodium single atoms compared to the conventional loading. They discovered that the deformed surface significantly increases the migration energy barrier of rhodium compared to the original surface, suppressing migration and aggregation.
Furthermore, the team found that not only rhodium but also platinum, iridium, and ruthenium-based single atoms can be loaded in high amounts on the deformed surface. Additionally, catalyst efficiency evaluation at the operating voltage required for the urea oxidation reaction showed hydrogen generation at a lower voltage than commercial electrodes, maintaining long-term stability for 100 hours without structural changes. Notably, this method saves about 16% of energy compared to water electrolysis, which produces hydrogen by splitting water at high voltage.
Hydrogen, spotlighted as an eco-friendly future fuel, exists chemically in water and fossil fuels, so it must be extracted from these sources. The only green hydrogen production technology that can extract hydrogen without carbon dioxide emissions is water electrolysis. However, due to the slow oxygen evolution reaction, very high voltage is required, resulting in low efficiency. Therefore, research on ammonia or urea oxidation reactions, which can produce hydrogen at low voltage, is active. Especially, unlike ammonia, which is gaseous at atmospheric pressure, urea is solid at room temperature, making it easier to transport and store, thus having high value as a hydrogen source.
Deputy Director Lee said, “We solved the long-standing problem of high loading and stabilization in the field of single-atom catalysts through a highly efficient urea oxidation electrocatalyst.” He added, “It is expected that high-purity hydrogen can be produced at a low cost and in an eco-friendly way.”
The research results were published online on the 30th of last month in the international journal on environment and energy, Energy & Environmental Science (IF 38.532).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.