"Inhalation-type RekkiRona Development Also Underway"
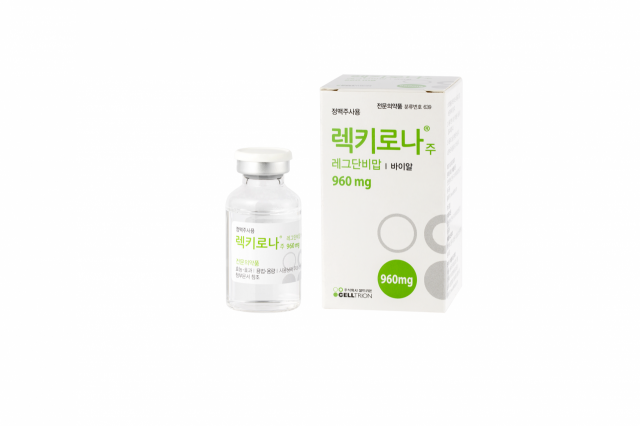
[Asia Economy Reporter Kim Ji-hee] Celltrion announced on the 16th that its COVID-19 antibody treatment 'Rekkirona' has confirmed efficacy in animal efficacy tests against the Delta variant virus.
Celltrion administered Rekkirona to 49 experimental mice infected with the Delta variant, and as a result, the viral titer significantly decreased compared to the control group that did not receive the drug, and clinical improvement effects such as protection against weight loss were confirmed. In particular, even at doses lower than the human therapeutic dose, the virus elimination efficacy was proven. In the case of experimental mice, the survival rate of the Rekkirona-administered group was 100%, whereas the control group showed a survival rate of 0%.
Accordingly, Celltrion confirmed efficacy against the Brazil-origin ‘Gamma’ variant, the South Africa-origin ‘Beta’ variant, and the India-origin ‘Delta’ variant. The company explained that this result means that even if Rekkirona’s neutralizing ability decreases at the cellular level, sufficient therapeutic efficacy can be exerted in vivo.
Celltrion is continuing to conduct cellular-level neutralization and animal efficacy tests of Rekkirona against variant viruses that are sporadically spreading worldwide and have the potential to evolve into dominant strains, in collaboration with reputable domestic and international institutions. Regarding the Lambda variant spreading in Peru, South America, neutralization results using pseudovirus (similar virus) have already been obtained. It was confirmed that the neutralizing ability is stronger than that of the existing Beta, Gamma, and Delta variants.
Meanwhile, Celltrion is also accelerating the development of an inhalable COVID-19 antibody treatment. It has started developing inhalable Rekkirona in collaboration with Inhalon Biopharma, a U.S.-based biotech company holding related patents and technology. Inhalon submitted data to the Australian ethics committee in June to initiate Phase 1 clinical trials of inhalable Rekkirona. The efficacy will be confirmed in Phase 2 clinical trials.
Inhalable Rekkirona delivers antibodies directly to the airway mucosa infected with the COVID-19 virus. It is expected to improve convenience and accessibility for COVID-19 patients by allowing easy administration of Rekkirona at home through an inhaler.
A Celltrion official said, "We have proven the efficacy of Rekkirona against major variants that have spread so far, including the globally spreading Delta and Lambda variants. We will continue to evaluate neutralizing ability against future variants and do our best to develop inhalable Rekkirona to provide various treatment options for COVID-19 patients."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










