Professor Jeong Yuseong's KAIST Research Team Successfully Elucidates High-Activity Platinum Wire Hydrogen Evolution Mechanism
[Asia Economy Reporter Kim Bong-su] The research team led by Professor Yoo-sung Jeong of the Department of Bio and Chemical Engineering at the Korea Advanced Institute of Science and Technology (KAIST) announced on the 29th that they have succeeded in elucidating the hydrogen evolution mechanism of highly active platinum wires through deep learning.
Platinum is an important catalyst used in fuel cells for electric vehicles and for obtaining hydrogen through water electrolysis, but its high cost has been a barrier to technology dissemination. Recently, studies synthesizing platinum into sawtooth wire shapes to save about ten times the amount of platinum have caused a significant stir, but the mechanism had not yet been clarified.
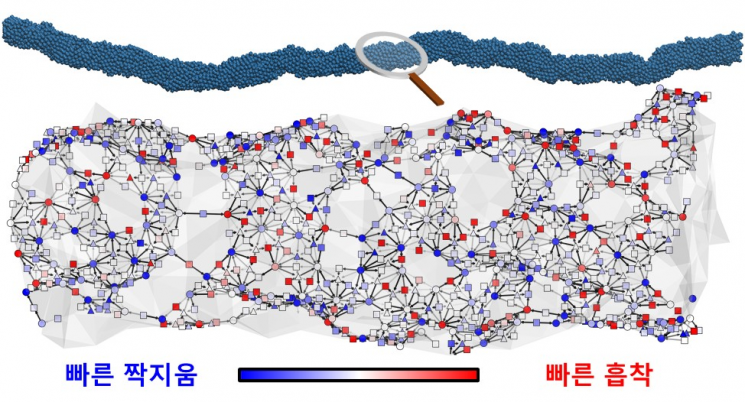
The research team devised deep learning methods to rapidly predict the properties of complex catalyst surfaces, and this time applied them to sawtooth platinum wires to elucidate the mechanism of the catalyst's high hydrogen activity. This is a new mechanism that breaks the conventional catalyst intuition. Previously, hydrogen evolution was known to occur in two steps: an adsorption reaction where protons are taken from water and hydrogen is adsorbed, and a coupling reaction where adsorbed hydrogen atoms combine to form hydrogen molecules. These two reactions were generally understood to occur at the same reaction site.
However, according to the newly discovered mechanism, on the sawtooth platinum surface, due to its uneven structure, there are separate reaction sites where the adsorption reaction occurs well and where the coupling reaction occurs well, and the synergistic effect of these two sites increases the catalytic activity by more than 400%. This concept, similar to improving work efficiency through division of labor, also exists in the molecular world.
Professor Yoo-sung Jeong said, "Although concepts of improving overall reaction efficiency through division of labor at the molecular level have existed before, this is the first time that a division of labor phenomenon according to structure has been elucidated in a single-component platinum catalyst," adding, "It is meaningful in that it presents a new perspective and design principle to enhance catalyst efficiency by changing the structure of single-component catalysts."
The research results were published online on the 17th of last month in the Journal of the American Chemical Society, an international journal published by the American Chemical Society.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.