At Expert Advisory Meeting: "Foreign Substances Unlikely to Significantly Affect Vaccine Efficacy"
[Asia Economy Reporter Kim Ji-hee] Amid controversy over foreign substances found in low dead space (LDS) syringes used for COVID-19 vaccinations, the Ministry of Food and Drug Safety (MFDS) has expressed through expert meetings that "the likelihood of foreign substances entering the human body is low." Additionally, it confirmed that the foreign substances reported in the syringes were unable to pass through the needle and remained inside the syringe.
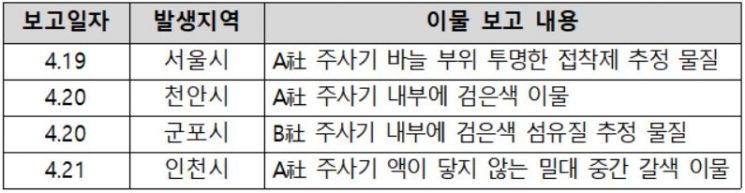
The MFDS announced on the 22nd that it received four reports of foreign substances occurring in LDS syringes used for COVID-19 vaccinations, conducted on-site inspections on the 21st, and held expert advisory meetings to assess potential harm to the human body. The foreign substances were discovered during pre-inspection checks before vaccinations at regional vaccination centers. To investigate the cause of the foreign substances, the MFDS immediately conducted on-site inspections at two manufacturing sites. Furthermore, to minimize the risk of contamination during the manufacturing process, the MFDS ordered strengthened management and preventive measures regarding the working environment, contamination control, and visual inspections.
At the expert advisory meeting, experts assessed the potential harm to humans, stating, "Although the likelihood of foreign substances entering the human body through the syringe is low, it is advisable to respond by considering even the slightest possibility for public safety." They also pointed out that "if foreign substances do enter the body, various adverse reactions may occur, so strict quality control during production and sufficient caution at vaccination sites are important," while mainly expressing the opinion that "the impact of foreign substances on vaccine efficacy is likely minimal."
However, regarding the syringes in which foreign substances were found, the opinion was that "considering the frequency of foreign substance occurrence and potential harm comprehensively, problematic individual products should be discarded under the current circumstances, and if the issue persists, stronger management measures will be necessary."
The MFDS plans to strengthen inspections to prevent contamination during the manufacturing process in the future. In cooperation with the Korea Disease Control and Prevention Agency (KDCA), it will ensure that vaccination centers check syringes for abnormalities before vaccination according to the ‘COVID-19 Vaccination Manual’ and establish a ‘hotline’ with the KDCA to promptly share relevant information to avoid disruptions in vaccination. The MFDS stated, "Considering that most domestic syringe manufacturers are small and medium-sized enterprises, we will prepare recurrence prevention measures with related ministries, including providing technical support for process management of vaccination syringe manufacturers through a public-private consortium with domestic private companies that have excellent process and quality control."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)













