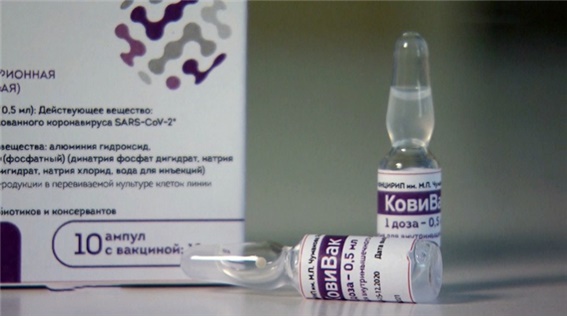
[Asia Economy Reporter Jang Hyowon] HumanN recently invested 7 billion KRW in MP Corporation (MPC), which is reportedly in discussions with the Russian Chumakov Institute of Poliomyelitis and Viral Encephalitides (hereafter Chumakov) regarding the domestic contract manufacturing and distribution rights of the COVID-19 vaccine CoviVac.
Konstantin Chernov, Deputy Director of Russia's Chumakov Institute, who visited Korea on the 23rd of last month, stated, "It is very risky for companies without vaccine development experience to develop and sell COVID-19 vaccines."
He predicted that the thrombotic side effects seen with AstraZeneca and Janssen vaccines are just the beginning, and that all vaccines, including Pfizer and Moderna, will eventually face controversy over side effects.
According to an industry insider, systematic research on viruses in Russia began in 1945 by Mikhail Chumakov. In 1953, Chumakov participated in the development of the polio vaccine led by the United States and the Soviet Union. He introduced and successfully mass-produced the vaccine developed by Albert Sabin (an American bacteriologist), ultimately eradicating the polio epidemic.
Established in 1957, Chumakov has been researching vaccines for over 60 years. In addition to the polio vaccine, it has developed and mass-produced vaccines for rabies, tick-borne encephalitis, cytomegalovirus infection, hepatitis A, and yellow fever. Notably, the yellow fever vaccine, essential for travel to African regions, supplies about 40% of the global demand through UNESCO.
With approximately 900 employees, Chumakov's strength lies not only in vaccine development but also in owning factories for vaccine production, allowing concurrent research on vaccine manufacturing processes. It conducts research on over 1,000 viruses and develops and produces vaccines, and based on this expertise, successfully mass-produced a COVID-19 vaccine in March.
Russian TV channel 'NTV' reported that CoviVac demonstrated immunogenicity and non-toxicity in animal trials and completed phases 1 and 2 clinical trials in February, with phase 3 clinical trials involving about 3,000 volunteers starting in March. Additionally, Russian media Izvestiya reported that CoviVac shows high immune efficacy against COVID-19 and particularly excellent effectiveness against variant viruses.
Aydar Ishmukhametov, Director of Research at Chumakov, said in an interview with TV channel 'Russia 24,' "CoviVac is characterized by being based on an inactivated coronavirus with no serious side effects or other risk factors detected during clinical trials," adding, "Perhaps people might contract unwanted diseases due to vaccines."
Moreover, CoviVac can be stored in a regular refrigerator at 2?8 degrees Celsius, eliminating the need for a cold chain during vaccine transportation. The second dose is administered 2?3 weeks after the first, and immunity against COVID-19 develops within 28 days, with antibody formation speed significantly faster than AstraZeneca's vaccine.
Meanwhile, the industry expects CoviVac to accelerate the timing of herd immunity amid the global vaccine supply shortage, including in Korea.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














