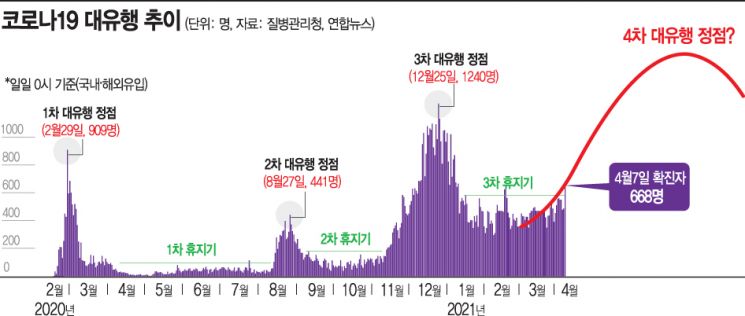
Similar Patterns with the Start of the 3rd Major Wave
National Infection Reproduction Number Exceeds 1
Possibility of Strengthening Social Distancing Gains Weight
[Asia Economy Reporters Jihee Kim and Chunhee Lee] The number of new COVID-19 cases surged by nearly 200 in a single day, raising concerns about a potential 4th wave of the pandemic. Experts warn that if the current spread is not contained, the crisis could surpass the 3rd wave, which saw over 1,000 cases per day. Although the introduction of the Janssen vaccine, following AstraZeneca and Pfizer, is becoming more tangible as a 'game changer,' there is still uncertainty about whether the overall supply will proceed as planned.
◆"Entering the 4th Wave" - Will Social Distancing Be Raised?= On the 7th, new COVID-19 cases rapidly increased to 668, leading experts to describe the situation as a 'deja vu of the 3rd wave.' Professor Woojoo Kim of Korea University Guro Hospital's Infectious Diseases Department said, "It appears that the situation from about four months ago during the 3rd wave is repeating," adding, "The health authorities' diagnosis on whether a 4th wave is underway has been inconsistent, and despite previous advice to implement short but intense quarantine measures, delayed responses have failed to curb the spread, repeating past mistakes."
Particularly, signs similar to those at the start of the 3rd wave are emerging, increasing anxiety. Currently, the infection reproduction number exceeds 1 in all regions, indicating an expansion phase of the outbreak. This is the second time since mid-December last year that the index has surpassed 1 across all regions. The recent spread is not centered on specific cluster infections but is linked to everyday settings such as various small gatherings, workplaces, churches, and entertainment venues, which is also a cause for concern. Additionally, other risk factors such as increased springtime mobility and the spread of variant viruses remain, providing ample room for case numbers to continue rising.
Attention is focused on the government's social distancing adjustment plan to be announced on the 9th. Since the country has been under level 2.5 social distancing for nearly a month and the recent spread is serious, there is considerable analysis that health authorities may consider raising the level. Prime Minister Se-kyun Chung stated at the Central Disaster and Safety Countermeasure Headquarters meeting, "The government will mobilize all means to prevent the 4th wave," adding, "We will closely monitor the situation and carefully review quarantine measures after next week, while vigorously considering effective additional measures."
◆Approval of Single-Dose Janssen Vaccine Imminent= The Ministry of Food and Drug Safety held a final review committee meeting on the morning of the same day to approve the single-dose Janssen (a subsidiary of Johnson & Johnson) COVID-19 vaccine, with results to be announced in the afternoon. The ministry conducts a three-tier advisory process before approving COVID-19 vaccines and treatments, involving a verification advisory group, the Central Pharmaceutical Affairs Committee, and the final review committee. Previously, the verification advisory group and the Central Pharmaceutical Affairs Committee recognized the preventive efficacy of the Janssen vaccine and advised that approval was possible.
The Janssen vaccine is a 'viral vector vaccine' manufactured by inserting the gene for the COVID-19 virus surface antigen into an adenovirus template. Among the COVID-19 vaccines scheduled for domestic introduction, it is the only one developed for a single-dose regimen. The vaccine showed preventive efficacy of 66.9% at 14 days and 66.1% at 28 days after administration. The immune response was maintained up to 12 weeks. Monitoring beyond this period has not yet been conducted.
However, the schedule for Janssen vaccine introduction is not confirmed beyond the plan to import doses for 6 million people in the second quarter. Unlike AstraZeneca and Pfizer vaccines, which were approved after detailed import schedules were set, Janssen vaccine is already in use overseas, and securing supply appears to be challenging. Yoojin Jung, head of the vaccine import team at the COVID-19 Vaccination Response Task Force, said, "Compared to AstraZeneca or Pfizer, Janssen vaccine has the difference of already being approved and used overseas," and assured efforts to avoid supply disruptions.
Concerns are also rising that AstraZeneca vaccine supply may become difficult due to export suspensions from India. The government explained that it is reviewing "all possible measures," including export bans on vaccines produced at the SK Bioscience plant in Andong.
 On the 2nd, a medical staff member at Mapo-gu Public Health Center in Seoul is busy filling syringes with the AstraZeneca COVID-19 vaccine ahead of vaccination for health and medical organization leaders. Photo by Hyunmin Kim kimhyun81@
On the 2nd, a medical staff member at Mapo-gu Public Health Center in Seoul is busy filling syringes with the AstraZeneca COVID-19 vaccine ahead of vaccination for health and medical organization leaders. Photo by Hyunmin Kim kimhyun81@
◆Adverse Reaction Reporting Rate Slightly Over 1%= The Central Disease Control Headquarters reported that as of midnight on the same day, 74 new cases of adverse reactions following COVID-19 vaccination were reported. Among them, two additional deaths were reported, bringing the total number of deaths to 38. Of the two deaths, one was after AstraZeneca vaccination and the other after Pfizer vaccination. Causality between vaccination and death has not yet been confirmed. One new suspected case of severe systemic allergic reaction, anaphylaxis, was also reported. The case involved an AstraZeneca vaccine recipient, and health authorities will evaluate causality through epidemiological investigation. The remaining 71 cases involved relatively mild symptoms such as muscle pain, headache, fever, chills, and nausea.
Since vaccinations began on February 26, a total of 11,215 suspected adverse reaction reports have been accumulated, representing about 1.05% of the 1,072,480 people who have received their first and second doses domestically. Reports related to AstraZeneca vaccine accounted for 10,674 cases, or 95.2% of all reports. Pfizer vaccine-related reports numbered 541 (4.8%). Among 887,452 AstraZeneca vaccine recipients and 185,028 Pfizer vaccine recipients, the adverse reaction reporting rates were 1.20% for AstraZeneca and 0.29% for Pfizer.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.