[Asia Economy Reporter Lee Chun-hee] The Janssen (a subsidiary of Johnson & Johnson) COVID-19 vaccine, which shows preventive effects with just one dose, has passed the second verification by the Ministry of Food and Drug Safety (MFDS).
On the afternoon of the 1st, Seo Kyung-won, Director of the Food and Drug Safety Evaluation Institute at MFDS, stated in a briefing, "It was advised that it is appropriate to approve a single-dose regimen for those aged 18 and over," and that the Central Pharmaceutical Affairs Deliberation Committee meeting held that day advised that the Janssen vaccine could be granted marketing authorization.
Before approving COVID-19 vaccines and treatments, the MFDS conducts a three-tier advisory review process involving the Verification Advisory Group, the Central Pharmaceutical Affairs Deliberation Committee, and the Final Inspection Committee. Previously, at the Verification Advisory Group meeting on the 29th of last month, it was also judged that the preventive effect of the Janssen vaccine could be recognized for approval.
The Central Pharmaceutical Affairs Deliberation Committee meeting included 17 external experts: 13 standing members of the Biologics Subcommittee, which is a specialized subcommittee for reviewing vaccine safety and efficacy, 3 members of the Verification Advisory Group, and 1 expert recommended by the Korean Medical Association. Additionally, 8 internal MFDS members from the ‘COVID-19 Crisis Response Support Headquarters’ vaccine review teams?comprising the overall review team, clinical review team, and quality review team?attended.
The Janssen vaccine is a ‘viral vector vaccine’ manufactured by inserting the gene for the surface antigen of the COVID-19 virus into an adenovirus vector. Among the COVID-19 vaccines scheduled for domestic introduction, it is the only one developed for a single-dose administration. Six million doses are expected to be introduced in the second quarter. However, the specific introduction schedule has not yet been finalized.
After reviewing clinical data submitted by Korea Janssen, the Central Pharmaceutical Affairs Deliberation Committee judged that the preventive effect of the Janssen vaccine is acceptable. According to Janssen’s clinical trial results, 116 out of 19,630 vaccinated individuals were confirmed COVID-19 positive 14 days after administration, compared to 348 out of 16,915 in the control group, showing a 66.9% prevention rate. Additionally, a 66.1% preventive effect was observed 28 days after administration.
Regarding long-term effects, it was recommended to continue tracking data on long-term efficacy. Current clinical data show that the immune response from the Janssen vaccine is maintained up to 12 weeks. Monitoring beyond this period has not yet been conducted.
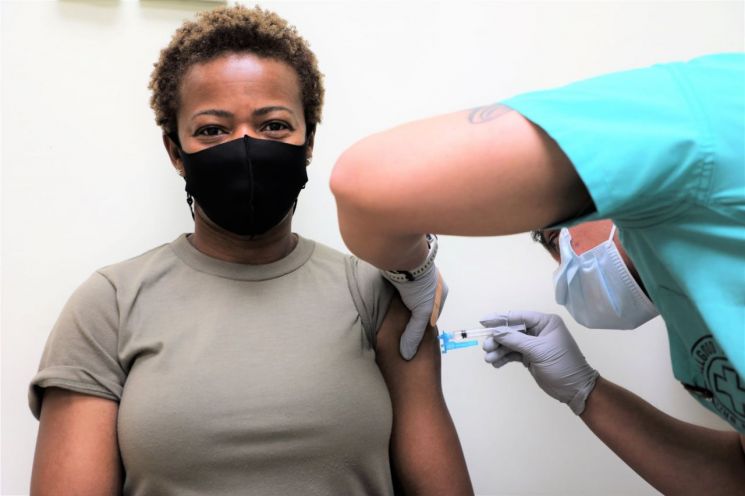 On the 11th of last month, Lieutenant Colonel Juliet Morarives, an emergency treatment nurse and the first person to receive the Janssen COVID-19 vaccine within the US Forces Korea, is being vaccinated. [Image source=Brian Olgut Hospital Facebook page] [Image source=Yonhap News]
On the 11th of last month, Lieutenant Colonel Juliet Morarives, an emergency treatment nurse and the first person to receive the Janssen COVID-19 vaccine within the US Forces Korea, is being vaccinated. [Image source=Brian Olgut Hospital Facebook page] [Image source=Yonhap News]
The Central Pharmaceutical Affairs Deliberation Committee also judged the safety of the Janssen vaccine to be at an acceptable level. Previously, the Verification Advisory Group noted that most local and systemic reactions after vaccination disappeared within three days, and the incidence and severity in the elderly were lower compared to adults. However, during clinical trials, seven cases of ‘serious adverse drug reactions’ possibly related to the vaccine were reported: one case each of Guillain-Barr? syndrome, pericarditis, brachial neuritis, post-vaccination syndrome, hypersensitivity reaction, and two cases of facial paralysis. The MFDS explained, "(These cases) were mostly in recovery at the time of clinical trial data submission."
Regarding this safety profile, the Central Pharmaceutical Affairs Deliberation Committee suggested that it is appropriate to additionally monitor and evaluate adverse events observed during clinical trials through a ‘Risk Management Plan’ after approval.
The MFDS plans to soon hold the Final Inspection Committee, the third advisory stage. Since the MFDS has announced a significant reduction in the COVID-19 vaccine approval and review period from the previous six months to within 40 days, it is expected that the formal approval decision for the vaccine will be made in early next month. Previously, the AstraZeneca vaccine received formal approval 37 days after the application was submitted, and the Pfizer vaccine was approved in 39 days.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.