 Choi Eun-hwa, Chairperson of the Vaccination Expert Committee (center), is explaining cases of blood clotting disorders found after AstraZeneca COVID-19 vaccination during the regular briefing on the domestic occurrence of COVID-19 and vaccination status held at the Korea Disease Control and Prevention Agency in Cheongju, Chungbuk, on the afternoon of the 22nd. On the left is Jung Eun-kyung, Commissioner of the Korea Disease Control and Prevention Agency (Director of the Central Disease Control Headquarters).
Choi Eun-hwa, Chairperson of the Vaccination Expert Committee (center), is explaining cases of blood clotting disorders found after AstraZeneca COVID-19 vaccination during the regular briefing on the domestic occurrence of COVID-19 and vaccination status held at the Korea Disease Control and Prevention Agency in Cheongju, Chungbuk, on the afternoon of the 22nd. On the left is Jung Eun-kyung, Commissioner of the Korea Disease Control and Prevention Agency (Director of the Central Disease Control Headquarters). [Photo by Yonhap News]
[Asia Economy Reporter Lee Chun-hee] The government's expert advisory body, the Vaccination Expert Committee, has recommended that "vaccination with the AstraZeneca COVID-19 vaccine should continue." Authorities have tentatively concluded that there is no causal relationship with newly reviewed post-vaccination death cases, while acknowledging causality with vaccination in two severe cases.
Choi Eun-hwa, Chairperson of the Vaccination Expert Committee (Professor at Seoul National University College of Medicine), stated at the regular briefing of the Central Disease Control Headquarters on the 22nd, "Given the ongoing risk from the COVID-19 pandemic in the current domestic situation, we recommend continuing vaccination with the AstraZeneca vaccine." She added that this position aligns with those of the World Health Organization (WHO), the European Medicines Agency (EMA), and the UK Medicines and Healthcare products Regulatory Agency (MHRA).
Chairperson Choi emphasized, "The AstraZeneca vaccine is an effective and safe vaccine that reduces severe infections and mortality caused by COVID-19, both in clinical trials and real-world medical settings," and added, "So far, data have not found any association between the AstraZeneca vaccine and an increased risk of blood clot formation."
The Vaccination Expert Committee held a meeting on the 20th to review recent reports of thrombotic adverse reactions related to the AstraZeneca vaccine both domestically and internationally, concluding that there is no link between vaccination and blood clot formation. They explained that thrombosis such as deep vein thrombosis and pulmonary embolism can naturally occur due to various causes, including COVID-19 infection, and are relatively common symptoms. Chairperson Choi also noted, "Cases of thrombosis observed after AstraZeneca vaccination have been reported at levels lower than the usual occurrence."
 On the afternoon of the 15th, medical staff demonstrated the preparation of the AstraZeneca vaccine at the COVID-19 vaccination center set up in the auditorium of Seongdong-gu Office in Seoul. Photo by Mun Ho-nam munonam@
On the afternoon of the 15th, medical staff demonstrated the preparation of the AstraZeneca vaccine at the COVID-19 vaccination center set up in the auditorium of Seongdong-gu Office in Seoul. Photo by Mun Ho-nam munonam@
However, the Vaccination Expert Committee stated that detailed investigations are needed regarding the causal relationship between the vaccine and very rare occurrences of disseminated intravascular coagulation (DIC) and cerebral venous sinus thrombosis (CVST), which occur at a frequency of about one case per one million people. Analysis of 20 million AstraZeneca vaccine doses administered in the UK and European Union (EU) countries reported 7 cases of DIC and 18 cases of CVST.
The committee explained that these two conditions differ from common thrombosis and can very rarely occur due to various causes, including COVID-19 infection. Chairperson Choi said, "Both conditions occur very rarely, at a frequency of about one case per one million people," and added, "The causal relationship with the AstraZeneca vaccine is not yet clear, but since these cases appear to be reported more frequently after AstraZeneca vaccination than generally expected, detailed investigations into causality are necessary."
Nevertheless, Chairperson Choi repeatedly emphasized, "These are extremely rare and unusual cases," and "The benefits of vaccination with the AstraZeneca vaccine far outweigh the potential risks." Accordingly, she urged the public, saying, "The Vaccination Expert Committee strongly recommends that eligible individuals do not delay and receive the COVID-19 vaccine promptly."
Authorities plan to continue evaluating the potential for blood clot formation related to the AstraZeneca vaccine. Park In-sook, Director of the Biologics Evaluation Division at the Ministry of Food and Drug Safety, explained, "Currently, the Ministry of Food and Drug Safety is requesting adverse reaction data related to vaccination from AstraZeneca worldwide," and "We have also requested detailed data, including individual adverse reaction reports and available data reviewed by regulatory agencies such as EMA, the UK MHRA, and the German regulatory authority, regarding blood clot cases." She added, "Once domestic and international safety information accumulates, we plan to comprehensively assess safety with expert consultation," and "If necessary, we will add information to the product labeling."
The Vaccination Damage Investigation Team also disclosed the results of reviewing 13 adverse reaction cases, including 3 death cases (2 previously reported deaths and 1 new death) and 10 severe cases including anaphylaxis, on the same day.
The investigation team tentatively concluded that all three death cases reviewed on that day were more likely caused by sepsis and pulmonary embolism, making it difficult to recognize a causal relationship between adverse reactions and death. Thus, among the 16 death cases under evaluation, causality between vaccination and death was not recognized in all 15 cases reviewed. One case is currently undergoing autopsy at the National Forensic Service.
Among the 10 cases reported as suspected anaphylaxis and severe cases, the investigation team acknowledged causality with vaccination in two cases. One case showed clinical symptoms consistent with anaphylaxis within 10 minutes, and the other case involved high fever and seizures after vaccination, followed by hypotension the next day. Authorities reported that symptoms in both cases have since improved.
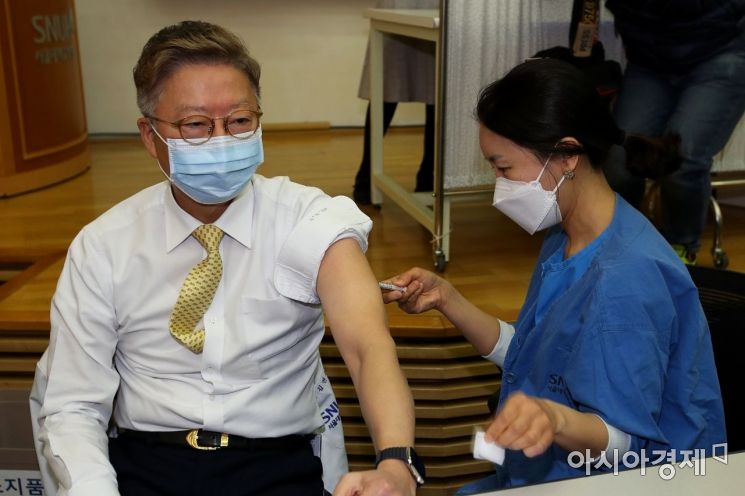 On the 4th, COVID-19 AstraZeneca vaccinations were administered to medical staff in a lecture room at Seoul National University Children's Hospital in Jongno-gu, Seoul. Kim Yeon-su, director of Seoul National University Hospital, is receiving the vaccine. 20210304 / Photo by Joint Press Corps
On the 4th, COVID-19 AstraZeneca vaccinations were administered to medical staff in a lecture room at Seoul National University Children's Hospital in Jongno-gu, Seoul. Kim Yeon-su, director of Seoul National University Hospital, is receiving the vaccine. 20210304 / Photo by Joint Press Corps
Meanwhile, starting from the 23rd, vaccination will begin nationwide for hospitalized, admitted, and staff aged 65 and older at nursing hospitals and facilities. The consent rate for vaccination was 76.9%, with 288,365 consenting out of 375,061 eligible individuals. Among nursing hospitals starting vaccination on the 23rd, 154,989 out of 205,983 eligible individuals (75.2%) consented, and among nursing facilities and mental health rehabilitation facilities starting vaccination on the 30th, 133,376 out of 169,078 eligible individuals (78.9%) consented.
Regarding concerns that the consent rate is somewhat lower compared to other vaccination groups, Jeong Eun-kyung, Director of the Korea Disease Control and Prevention Agency, responded, "Staff members still show a high vaccination rate, and for hospitalized·admitted individuals, underlying diseases, health status, and age factors are considered," adding, "We believe that differences in vaccination and consent rates also reflect public anxiety." She further explained, "We will provide detailed information and thorough guidance so that the public can make accurate decisions," and said this would help improve vaccination rates.
The COVID-19 Vaccination Response Task Force plans to stagger vaccination schedules to avoid concentration of vaccinations in a short period, considering the availability of nursing and care personnel. Nursing hospitals aim to complete vaccination within two weeks instead of the previous five days, and nursing facilities aim to complete vaccination within six weeks instead of the previous one month. In particular, for nursing facilities, where health center visiting teams or planned medical staff visit to administer vaccines, vaccination schedules will be arranged with intervals of 3 to 4 days between visits for two or more visits.
Additionally, during the vaccination process, pre-vaccination physicians will focus on safety when deciding whether to vaccinate individuals by assessing their health status on the day, and will avoid vaccinating those in poor health. They will also ensure thorough measurement and recording of vital signs before and after vaccination.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















