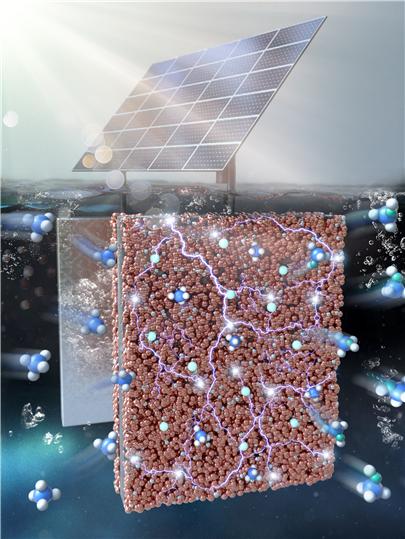
 Electrochemical conversion system of methane integrated with a solar cell. Methane is electrochemically oxidized to methanol on the surface of a copper oxide-based catalyst immersed in the electrolyte. This oxidation reaction efficiently occurs at room temperature under the potential applied by the solar cell. Image and caption provided by Professor Junhyuk Moon, Sogang University.
Electrochemical conversion system of methane integrated with a solar cell. Methane is electrochemically oxidized to methanol on the surface of a copper oxide-based catalyst immersed in the electrolyte. This oxidation reaction efficiently occurs at room temperature under the potential applied by the solar cell. Image and caption provided by Professor Junhyuk Moon, Sogang University.
[Asia Economy Reporter Kim Bong-su] Methane, known as one of the main culprits of global warming, has another face. It is also an 'energy resource' that can be reformed into useful chemicals such as ethylene and methanol, which are the main components of natural gas and shale gas. However, the process is complex and costly. Recently, domestic researchers have been actively conducting studies to develop environmentally friendly and highly efficient processes.
◇ Challenging Methane Conversion Process
Methane is a substance derived from petrochemical processes and shale gas. Of the 900 million tons of methane produced annually worldwide, 92.2% is used for heating or power generation, while only 7.8% is used as a chemical raw material. Scientists have been searching for nearly a century for ways to convert methane into chemical raw materials. However, catalytic processes that convert methane directly into chemical raw materials without oxygen input require very advanced technology and produce many by-products, preventing commercialization. In particular, methane is a stable molecule with high bond energy, so breaking its chemical bonds and initiating reactions requires high energy such as high temperatures.
◇ Development of Environmentally Friendly, Low-Cost Process Using Solar Energy
Professor Moon Jun-hyuk’s research team at Sogang University developed a technology that uses electrochemical catalysts to oxidize methane at room temperature and convert it into methanol. In other words, they discovered a process that uses solar energy to convert methane into methanol, a useful substance widely used as a chemical raw material, at room temperature without continuous energy consumption. Professor Moon explained, "High-temperature conditions can decompose the products again, making it difficult to obtain the desired products with high selectivity. Therefore, we considered technology to convert methane at low temperatures such as room temperature, and instead of developing highly active catalysts as many researchers have done, we attempted to apply other energy sources that replace thermal energy."
In this study, Professor Moon’s team synthesized mixed transition metal oxides as catalytic electrodes capable of electrochemical oxidation and integrated solar cells to apply potential to the catalytic electrodes, implementing a reaction system. They also controlled the composition of the transition metal oxide catalysts to identify the optimal catalyst composition that achieves the highest methane-to-methanol conversion rate and conducted isotope experiments to confirm the conversion reaction mechanism.
Professor Moon stated, "The main achievement of this study is proposing a 'zero-energy' system that converts methane using solar cells," adding, "Using transition metal oxide catalysts with high activity for electrochemical oxidation of methane, we achieved conversion rates surpassing those of chemical catalysts." He further added, "This technology is expected to become a model of environmentally friendly chemical processes that reduce methane, a climate change gas, while simultaneously converting it into high value-added chemical substances."
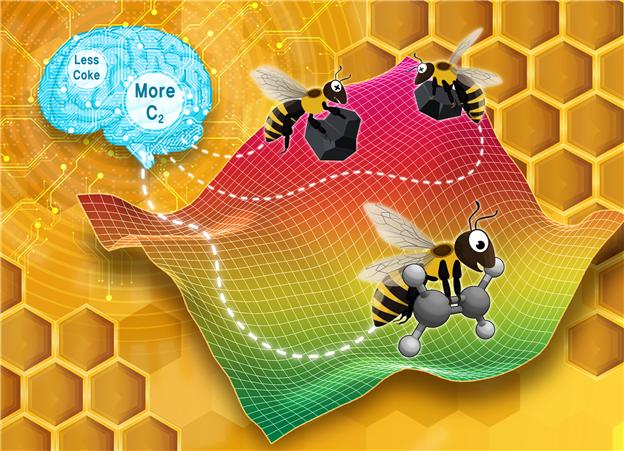 An illustration of the Artificial Bee Colony algorithm used to find conditions that maximize the yield of the desired product (C2 compound) while minimizing the formation of byproducts (charcoal, coke). (Back cover image of the Reaction Chemistry & Engineering journal)
An illustration of the Artificial Bee Colony algorithm used to find conditions that maximize the yield of the desired product (C2 compound) while minimizing the formation of byproducts (charcoal, coke). (Back cover image of the Reaction Chemistry & Engineering journal)
◇ Enhancing Efficiency Using Artificial Intelligence
There are also research teams that developed processes with higher efficiency using artificial intelligence. The teams led by Dr. Jang Hyun-joo and Dr. Kim Hyun-woo from the Chemical Platform Research Division and Dr. Kim Yong-tae from the Chemical Process Research Division at the Korea Research Institute of Chemical Technology are the protagonists. After conducting experiments under challenging conditions such as temperatures exceeding 1000 degrees Celsius, gas velocity, and pressure directly in the laboratory, they used data from 250 experiments as a basis for artificial intelligence to virtually perform over 10,000 simulations, increasing the yield (output relative to input) by more than 10% compared to previous results and successfully verifying this in the laboratory. Ethylene is called the 'rice' of petrochemicals and is the most widely used chemical in the industry. Ethylene serves as a raw material in most everyday items, from general plastics and vinyl to synthetic rubber, various construction materials, adhesives, and paints. Previously, in 2019, Dr. Kim Yong-tae’s team at the Korea Research Institute of Chemical Technology recorded a yield of 5.9% with almost no by-products, but through subsequent research and collaboration with artificial intelligence studies, they achieved a 13% yield, more than double the 2019 yield.
The basic yield for methane-to-ethylene conversion is generally expected to be above 25% in academia, with by-product selectivity (the ratio of by-products formed relative to converted methane) below 20%. Especially, if by-products (carbon deposits) accumulate in the process pipeline, it can cause environmental problems as well as safety issues such as explosions. The key is to increase yield while minimizing by-products. Currently, countries close to commercializing this technology include the United States, China, and South Korea.
The research team collected 250 reaction data points from direct laboratory experiments and trained a machine learning model. Using this model, artificial intelligence finely adjusted various conditions such as temperature, velocity, pressure, and reactor structure, generating over 10,000 virtual conditions and experimental results.
The team then applied the virtual experimental data to the artificial bee colony algorithm of artificial intelligence. In nature, bee colonies search for areas with honey, collect detailed information about where and how much honey is available, and identify the richest sources to gather honey. Similarly, the artificial bee colony algorithm explores various virtual experimental conditions, collects detailed information on which conditions yield what results, and makes decisions through three stages to find conditions that produce better experimental outcomes.
The research team identified experimental conditions that yield high output with minimal by-products using artificial intelligence and verified these within an error margin through direct experiments.
Dr. Jang Hyun-joo, head of the Chemical Platform Research Division, said, "In research fields with complex processes and many variables, we were able to find reaction conditions with high yield in a very short time through 250 experiments and the help of artificial intelligence," adding, "The artificial intelligence technology developed this time can find various chemical reaction conditions in virtual environments, so it is expected to be immediately applicable to many important reactions in the chemical industry in the future."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















