Novavax Additional 20 Million Doses Secured
Enough for 147% of Population to Achieve Herd Immunity
Phase 3 Trials Ongoing, Expected Completion in Q1
Possible Introduction as Early as Q2
First 50,000 Domestic Vaccine Doses Arriving Next Month
"High Possibility of First Dose for Medical Staff"
[Asia Economy Reporter Seo So-jeong] On the 20th, the government secured an additional 20 million doses of the novel coronavirus (COVID-19) vaccine from the U.S. company Novavax, bringing the total number of doses secured to 76 million, including the previously secured 56 million doses. This amount exceeds the level required to form herd immunity, corresponding to about 147% of the population.
With the big picture of vaccine procurement finally completed after many twists and turns, there are calls to now focus on rapid and safe vaccination. On the same day, as the number of COVID-19 confirmed cases remained at 404, showing signs of the 'third wave' subsiding, the challenge for health authorities is to start vaccinations without increasing the number of confirmed cases.
Traditional Vaccine Manufacturing Method... Expected to Complement Existing Vaccines
The government has so far completed purchase contracts with four multinational pharmaceutical companies: AstraZeneca for 10 million doses (20 million shots), Janssen for 6 million doses (6 million shots), Pfizer for 10 million doses (20 million shots), and Moderna for 20 million doses (40 million shots). Including the 20 million doses from Novavax and 10 million doses (20 million shots) supplied through the international project for joint vaccine purchase and distribution, the 'COVAX facility,' the total will increase to 76 million doses, enough for the entire population and more.
Experts expect the Novavax vaccine to complement the four vaccines previously secured by the government because it uses a manufacturing method similar to many influenza vaccines and differs from the others in its production process, potentially offsetting side effects. Unlike the four vaccines already contracted, Novavax's vaccine is made using the traditional vaccine manufacturing method called the 'recombinant protein subunit' approach. It induces an immune response by administering part of the antigen protein into the body. Notably, this vaccine has a long shelf life of 2 to 3 years and can be stored at room temperature between 2 to 8 degrees Celsius, which is an advantage. This reduces transportation burdens compared to vaccines like Pfizer's, which require ultra-cold storage at around minus 70 degrees Celsius.
Phase 3 Clinical Trials in the U.S. and U.K... Domestic Supply Possible as Early as Q2
However, the timing of supply is crucial. The ongoing Phase 3 clinical trials of the Novavax vaccine are expected to be completed in the first quarter. Novavax is currently conducting Phase 3 trials in the U.S., Mexico, and the U.K. It is anticipated to apply for emergency use authorization from the U.S. Food and Drug Administration (FDA) by March, and there is a high possibility that the vaccine will be introduced domestically as early as the second quarter. Australia and New Zealand have completed contracts for 25.5 million and 5.25 million doses respectively with Novavax, with Australia expected to receive initial supplies in the second to third quarter of this year.
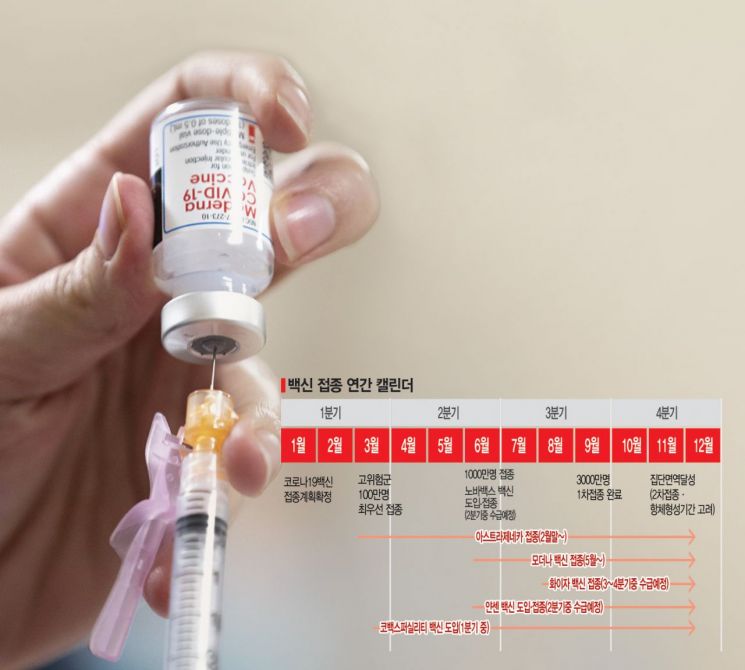
Ahead of the vaccination start next month, the government plans to sequentially open a management system for COVID-19 vaccination to accept advance reservations. The system is being revamped to provide accurate vaccination information and public services related to vaccination, with phased openings planned from February. Preparations are underway to allow eligible recipients to make reservations through the system and receive services such as guidance on vaccination schedules and locations, as well as issuance of vaccination certificates. Jeong Eun-kyung, Director of the Korea Disease Control and Prevention Agency, said, "We are supporting local governments with the Ministry of the Interior and Safety to ensure prompt preparation of vaccination centers and designation of entrusted medical institutions," adding, "We plan to finalize and announce the vaccination implementation plan by the end of this month."
The first domestic vaccinations are likely to take place in early to mid-February. Prime Minister Chung Sye-kyun appeared on MBC Radio on the same day and said, "Among the 10 million doses contracted through the COVAX facility, an initial batch of 50,000 doses may arrive in early February," adding, "We have been contacted about receiving them in early February, and we have responded affirmatively and are currently preparing." The Prime Minister also added, "The first vaccination targets are likely to be healthcare workers."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.