Observation of Carbon Dioxide Molecular Decomposition Process at the Ultrafine Scale
[Asia Economy Honam Reporting Headquarters Reporter Lee Gwan-woo] Professor Moon Bong-jin of Gwangju Institute of Science and Technology (GIST), Department of Physics and Optical Science, SRC Center for Ultra-Fast X-ray Science, C-AXS, announced on the 9th that he succeeded in directly observing the moment when carbon dioxide molecules decompose on the surface of a rhodium (Rh) catalyst.
This research was conducted in collaboration with the research team of Associate Director Park Jung-young (Professor of Chemistry at KAIST) from the Nano Materials and Chemical Reactions Research Group (Director Yoo Ryong) at the Institute for Basic Science (IBS), and the research team of Professor Kim Hyun-yoo (Department of Materials Science and Engineering) at Chungnam National University.
As damage caused by climate change increases every year, technologies to convert carbon dioxide, a greenhouse gas that accelerates global warming, into useful substances have recently been actively researched.
The research team’s results provided direct evidence of the chemical reaction that can remove carbon dioxide, the main culprit of global warming, and convert it into useful materials.
If captured carbon dioxide is converted into clean fuels such as methane or methanol, it could solve environmental and petroleum energy dependency issues.
The problem is that carbon dioxide (CO2) is chemically very stable, so a high amount of energy is consumed for its conversion. The initial process of decomposing carbon dioxide into carbon monoxide (CO) and oxygen (O) requires a high-pressure reaction reaching tens of atmospheres. Therefore, to design an optimal reaction pathway and improve conversion efficiency, it is important to closely understand the decomposition mechanism of carbon dioxide.
However, until now, only limited evidence such as spectroscopic analysis has been presented, and no research has accurately demonstrated the chemical mechanism of carbon dioxide decomposition at the atomic level.
The research team began the study to observe the carbon dioxide decomposition process in real time under actual reaction conditions. They drew inspiration from the theoretical prediction that carbon dioxide molecules, which are only a few angstroms (?·one ten-billionth of a meter) in size, can undergo structural changes on the catalyst surface if the internal pressure of the chemical reactor sufficiently increases.
Professor Kim Hyun-yoo of Chungnam National University explained, “The ambient pressure environment we live in is a high-pressure environment that supplies considerable energy from the perspective of the small carbon dioxide molecules. Due to the surrounding pressure, the frequency of molecular collisions per unit area increases dramatically, causing the molecules to become unstable and eventually decompose.”
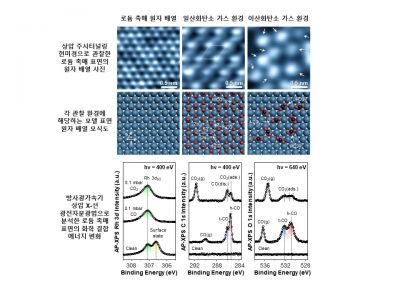
Subsequently, the research team used a synchrotron radiation accelerator, known as a ‘giant light microscope,’ to measure subtle changes in chemical bond energy on the rhodium catalyst surface. They confirmed that after the reaction started under ambient pressure conditions, the amount of carbon monoxide gradually increased.
They also discovered that the difference in electron cloud density of carbon dioxide undergoing structural changes was maximized on the rhodium catalyst surface. This provided evidence that the decomposition of carbon dioxide begins on the surface of the rhodium catalyst.
Professor Moon Bong-jin of GIST said, “To effectively remove and utilize carbon dioxide, which is pointed out as a cause of global warming, it is necessary to thoroughly investigate the decomposition mechanism of carbon dioxide. This research is significant in that it observed changes in surface carbon dioxide at the atomic level through joint research in experimental and computational science fields and proposed a standard research methodology for follow-up studies.”
Associate Director Park Jung-young of IBS said, “The theory that carbon dioxide decomposes spontaneously on the catalyst surface was proposed long ago, but direct experimental evidence had not been presented, making it a challenge for over 40 years. We plan to conduct research to identify the key links affecting the conversion rate of carbon dioxide in the future.”
Meanwhile, this research achievement was published online on the 6th at 7 p.m. in the international academic journal Nature Communications (IF 12.121).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















