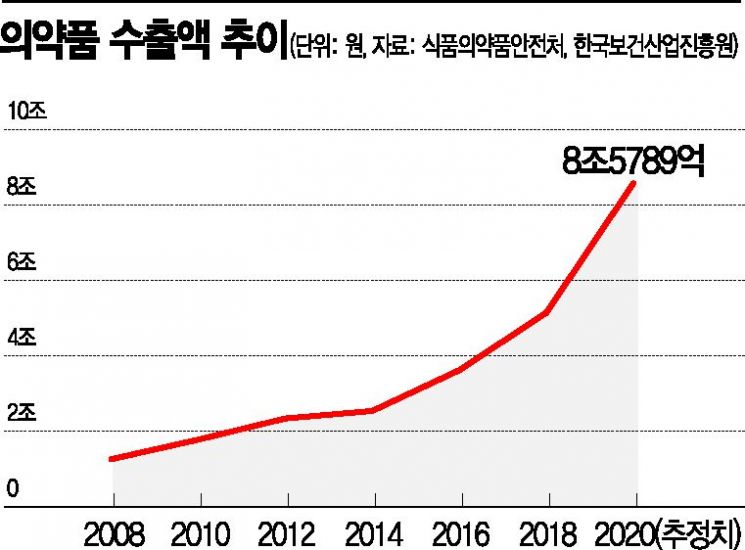
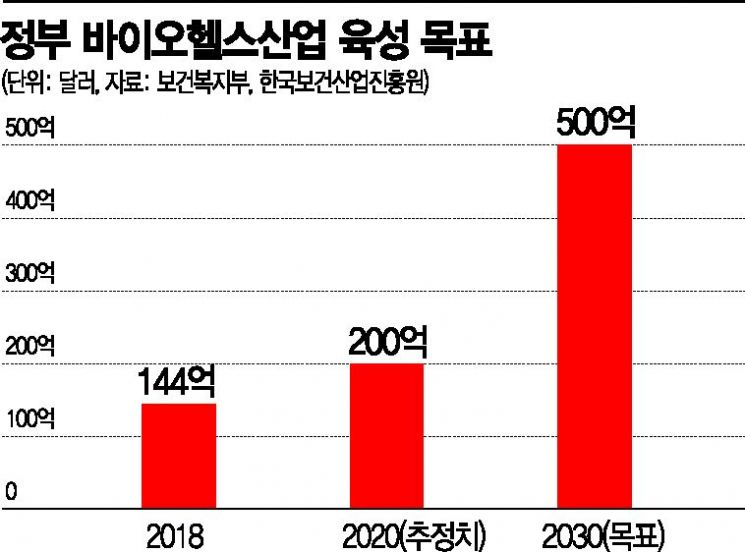
Pharmaceutical Exports Increase by 18.0% Annually Over 5 Years
Biohealth Target of $50 Billion by 2030
Over 50 Companies Developing COVID-19 Treatments and Vaccines
Diagnostic Reagents and Kits, K-Quarantine 'Trust'
Driving Health Industry Exports...Up 22% in Four Months
[Asia Economy Reporter Cho Hyun-ui] K-Bio is turning the crisis of the novel coronavirus infection (COVID-19) pandemic into an opportunity. Our companies took the initiative in developing diagnostic kits, receiving love calls from over 100 countries worldwide. While the number of companies developing therapeutics and vaccines was in the single digits during the 2015 Middle East Respiratory Syndrome (MERS) outbreak, it has now grown to about 50. The strong performance of Korea’s bio industry leaders, Samsung Biologics and Celltrion, in the global market has also elevated the status of K-Bio.
Jung Yoon-taek, head of the Pharmaceutical Industry Strategy Institute, said on the 15th, “Domestic pharmaceutical exports have steadily increased every year, driven by the success of biosimilars and others. The average annual growth rate over the past five years has reached 18.0%, and with the COVID-19 situation strengthening K-Bio’s status, it is expected to rise further.” He added that the government’s goal to expand the biohealth industry export scale from $14.4 billion in 2018 to $50 billion by 2030 is by no means unattainable. Shin Yoo-won, team leader at the Korea Health Industry Development Institute, also predicted, “As trust in K-quarantine products such as diagnostic kits has increased, the bio industry will continuously drive health industry exports.”
◆Rapid Growth of K-Bio’s Top Two= Celltrion and Samsung Biologics, the twin pillars of the domestic bio industry, delivered solid results despite the impact of COVID-19. As exports of biosimilars they produce have begun to increase significantly, their share in total pharmaceutical exports has risen to about half, double that of a year ago. Celltrion recorded first-quarter sales of 372.8 billion KRW, a 68% increase year-on-year, driven by strong sales of Remsima SC launched in Europe this year. Samsung Biologics posted 207.2 billion KRW in first-quarter sales, up 65.3% year-on-year, as its subsidiary Samsung Bioepis’s three biosimilars for autoimmune diseases exceeded $200 million in European sales.
Both companies are expected to continue their growth by expanding their product lines. Samsung Biologics’s subsidiary Samsung Bioepis has begun Phase 3 clinical trials for the Aflibercept biosimilar 'SB15,' expanding its ophthalmic disease product portfolio. Aflibercept is one of the most widely used treatments for macular degeneration worldwide, and no biosimilars have been approved yet. If Samsung Bioepis succeeds in clinical trials for SB15 following the Lucentis biosimilar 'SB11,' it will secure pipelines for two macular degeneration treatments. Samsung Biologics is also achieving significant results in the global biopharmaceutical contract manufacturing organization (CMO) market, having secured orders in the first half of this year nearly double last year’s sales.
Celltrion recently began global clinical trials for two follow-up biosimilars targeting asthma and autoimmune diseases, its main biosimilar products. Additionally, by acquiring patents and distribution rights for 18 products in the Asia-Pacific region from multinational pharmaceutical company Takeda, Celltrion is expanding its product range beyond biosimilars to include treatments for chronic diseases such as diabetes and hypertension, positioning itself as a global comprehensive pharmaceutical company.
◆Therapeutics Development Within This Year, Vaccines Targeted for Next Year= Currently, there are about 50 domestic companies developing COVID-19 therapeutics and vaccines. Considering there were only about seven during the MERS outbreak, this represents more than a sevenfold increase. According to the National Clinical Trial Support Foundation, 13 COVID-19 therapeutic clinical trials have been approved domestically. Excluding investigator-initiated trials conducted for academic purposes at hospitals, half of the six pharmaceutical company-led clinical trials are being conducted by domestic companies.
GC Green Cross is developing a plasma therapy in collaboration with the National Institute of Health, scheduled for completion this year, while Celltrion’s antibody therapy is expected to be completed next year. Vaccine development is targeted for next year, with SK Bioscience, GeneOne Life Science, and Genexine testing efficacy and safety at the preclinical stage. Jerome Kim, director-general of the International Vaccine Institute (IVI), said, “Vaccine development usually takes 5 to 10 years, but for COVID-19, it is expected to be drastically shortened to 6 to 18 months. Success in development would be unprecedented.”
Domestic diagnostic kits are also gaining global attention for their high accuracy and rapid diagnostic capability. Exports of domestic diagnostic kits surged from $3,400 in January to $201,233,500 in April. As diagnostic kits attract attention in the global market, health industry exports have also increased significantly. According to the Korea Health Industry Development Institute, domestic health industry exports reached $6.08516 billion from January to April this year, a 21.9% increase compared to the same period last year. This contrasts with the negative export trend in other sectors due to the COVID-19 impact. Team leader Shin explained, “Since March, COVID-19 diagnostic reagents and diagnostic kits have increased sharply, driving health industry exports.”
 On the 1st, at the screening clinic of Manan-gu Public Health Center in Anyang-si, Gyeonggi Province, a citizen who came with a child is waiting for a diagnostic test. Recently, nine church members and related personnel from churches located in Anyang and Gunpo, Gyeonggi Province, who traveled to Jeju, were confirmed positive for COVID-19. Among them, five are family members of church members from Church A located in Manan-gu, and it has been confirmed that a student from Yangji Elementary School in the district is also included. / Anyang ? Photo by Kim Hyun-min kimhyun81@
On the 1st, at the screening clinic of Manan-gu Public Health Center in Anyang-si, Gyeonggi Province, a citizen who came with a child is waiting for a diagnostic test. Recently, nine church members and related personnel from churches located in Anyang and Gunpo, Gyeonggi Province, who traveled to Jeju, were confirmed positive for COVID-19. Among them, five are family members of church members from Church A located in Manan-gu, and it has been confirmed that a student from Yangji Elementary School in the district is also included. / Anyang ? Photo by Kim Hyun-min kimhyun81@
◆“We Will Make Bio a New Growth Engine”= As K-Bio takes on a role driving the national economy, South Korea is preparing to establish itself as a next-generation bio powerhouse. The government plans to systematize infectious disease response procedures and related industries to develop the bio industry as a key export item. Hong Nam-ki, Deputy Prime Minister and Minister of Economy and Finance, stated, “In response to the COVID-19 crisis, we will actively foster the infectious disease response industry by intensively supporting research and development (R&D) through Phase 3 clinical trials for early development of therapeutics and vaccines to lead the global quarantine market.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.