 Schematic diagram of measuring hydrogen bonding energy of water at specific biological sites using probe molecules
Schematic diagram of measuring hydrogen bonding energy of water at specific biological sites using probe molecules
[Asia Economy Reporter Junho Hwang] Domestic researchers have developed a technology to measure the relationship between water and proteins in the human body. Water constitutes most of living organisms, and understanding the characteristics of water within the body can greatly aid in identifying the causes of diseases or developing new drugs, according to the research team. Ulsan National Institute of Science and Technology (UNIST) announced these findings on the 28th, based on the research results from Professor Oh-Hoon Kwon's team in the Department of Natural Sciences.
Measuring Hydrogen Bonds of Water in the Body Using Laser
 Research team of Professor Kwon Oh-hoon (first from the left) and Professor Kwak Sang-gyu (fifth from the left). Next to Professor Kwon Oh-hoon is the first author, researcher Park Won-woo.
Research team of Professor Kwon Oh-hoon (first from the left) and Professor Kwak Sang-gyu (fifth from the left). Next to Professor Kwon Oh-hoon is the first author, researcher Park Won-woo.
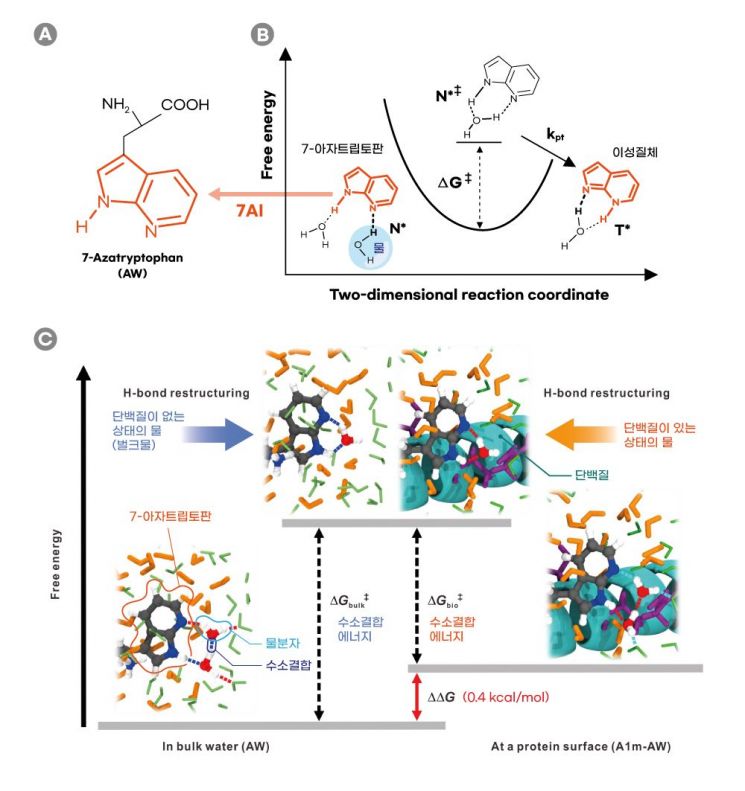
The research team developed a technology that measures the hydrogen bond energy of water inside the body by utilizing molecules that capture hydrogen ions (protons) from water when exposed to laser light.
Hydrogen bonding is a chemical bond created by the electrical attraction around molecules bonded to hydrogen. This bond plays a crucial role in connecting water molecules and determining the structure of biomacromolecules.
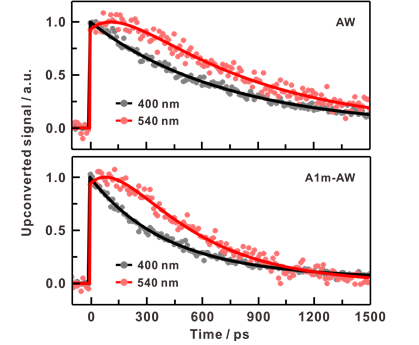
The team measured hydrogen bond energy by using a molecule called 7-azatryptophan, which, when excited by light, seeks hydrogen ions (H+) from surrounding water molecules. When this molecule captures hydrogen ions from water molecules, the hydrogen bonds among the surrounding water break and then rearrange. By observing the reaction speed of this process, the hydrogen bond energy of water molecules was inferred.
The substance 7-azatryptophan, in its excited state, steals hydrogen ions from neighboring water molecules and then returns its own hydrogen ions back to the water molecules. During this process, hydrogen bonds between water molecules break and rearrange. The stronger the hydrogen bonds between water molecules, the longer it takes to break them, resulting in a slower reaction speed.
The research team synthesized an artificial protein containing 7-azatryptophan to verify this. Using spectroscopy that measures the light emitted by 7-azatryptophan in its excited state at picosecond (one trillionth of a second) intervals, they calculated the reaction speed. The results showed that the hydrogen bond energy of water near proteins was lower than that in the absence of proteins. Water near proteins has weaker hydrogen bond energy, making it easier to break. This finding was consistent with results from computer simulations.
Tracking Water Characteristics in the Body to Identify Disease Causes
Wonwoo Park, the first author and a doctoral researcher in the Department of Chemistry at UNIST, explained, "By using an 'excitation-probe method' that combines a laser to excite 7-azatryptophan and a probe system that detects light emitted from reactants before hydrogen ion exchange and products formed after exchange, we were able to capture the moment the reaction occurs."
Professor Oh-Hoon Kwon said, "This study presents an experimental methodology to derive the hydrogen bond energy of water in specific regions within living organisms. It can be used to understand the structure and folding of biomacromolecules like proteins and to track the role of water in numerous biological phenomena such as protein-ligand binding, which will aid in new drug development."
Meanwhile, this research was conducted jointly with Professor Sangkyu Kwak's team from the Department of Energy and Chemical Engineering at UNIST and Professor Taehyun Yoo's team from the Department of Applied Biological Chemical Engineering at Ajou University (President Hyung-Joo Park). The research results were selected as a highlighted paper in the top-tier chemistry journal Angewandte Chemie and published as the cover article on the 27th.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.