22.5% Weight Loss Achieved in Phase 2 Trial
"Amylin Combination Therapy to Address Nausea Side Effects Draws Attention"
With Roche making a full-fledged entry into the global obesity drug market, which has been dominated by Eli Lilly and Novo Nordisk, signs are emerging of a major shift in the competitive landscape for obesity treatments. As the competition for powerful weight loss efficacy intensifies, Roche has put forward a combination strategy using an amylin analogue to address the most significant weakness of glucagon-like peptide-1 (GLP-1) class therapies: nausea as a side effect.
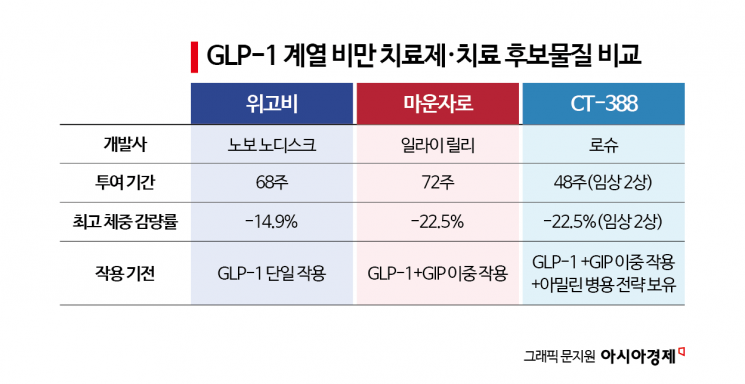
According to the pharmaceutical industry on January 29, Roche announced the previous day the results of its Phase 2 clinical trial for CT-388, a dual agonist obesity treatment targeting both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), and stated its intention to begin Phase 3 trials within the first quarter of this year. The Phase 2 trial involved 469 patients who were either obese or overweight. At the 48-week mark, the group receiving the highest dose of 24mg showed an average weight loss of 22.5% compared to placebo. This is similar to the maximum weight loss rate observed in the Phase 3 trial of Mounjaro (active ingredient: tirzepatide), which is considered the leading obesity treatment in the industry.
No early plateau in weight loss was observed with CT-388. Among patients receiving the highest dose, 95.7% lost at least 5% of their body weight, and 26.1% achieved weight loss of 30% or more. The treatment discontinuation rate was 5.9%, higher than the placebo group, but most gastrointestinal adverse reactions were mild or moderate in severity.
Roche's key differentiation strategy is focused on managing side effects. Building on its GLP-1/GIP dual agonist CT-388, Roche is pursuing a future combination strategy with the amylin analogue petrelintide. Amylin is a hormone secreted from the pancreas along with insulin after meals, which slows gastric emptying and increases satiety. While its action partially overlaps with GLP-1, the signaling pathways in the central nervous system are different. Roche believes that combining an amylin analogue can help distribute the appetite-suppressing effect more gradually compared to GLP-1 monotherapy, while also reducing the intensity of gastrointestinal side effects such as nausea. The aim is to induce weight loss without abrupt gastrointestinal stimulation, thereby improving treatment adherence.
Competitors are also paying attention to the amylin combination strategy. Novo Nordisk is developing a combination strategy that incorporates an amylin analogue as one way to maintain and enhance the weight loss effects of GLP-1 class therapies. By combining an amylin analogue, the approach stimulates different central appetite regulation pathways simultaneously. The goal is to achieve even greater weight loss compared to GLP-1 monotherapy.
Industry observers note that, despite being a latecomer, Roche is emerging as a game-changer in the obesity drug market by securing a pipeline that includes both injectable and oral formulations. In addition to CT-388, Roche also possesses CT-996, a GLP-1 candidate that can be administered orally.
While global pharmaceutical companies are expanding the obesity drug market by focusing on mechanism-based competition, domestic pharmaceutical companies are seeking differentiation by improving formulation technology and administration methods. Hanmi Pharmaceutical is targeting simultaneous weight loss and minimization of muscle loss with a long-acting triple agonist. Daewoong Pharmaceutical aims to improve dosing convenience and medication adherence through microneedle patch technology that increases bioavailability. Ildong Pharmaceutical is researching an orally administered GLP-1 class candidate as a treatment for obesity and metabolic diseases.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.