A method to enhance the performance of all-solid-state batteries solely through structural design has been devised. This approach is drawing attention as it could provide a breakthrough in addressing the longstanding challenge of simultaneously achieving safety, performance, and cost-effectiveness in all-solid-state batteries.
On January 7, KAIST announced that a research team led by Professor Donghwa Seo in the Department of Materials Science and Engineering, in collaboration with teams led by Professor Sungkyun Jung of Seoul National University, Professor Yunseok Jung of Yonsei University, and Professor Kyungwan Nam of Dongguk University, has developed a core material design method for all-solid-state batteries. This method uses low-cost raw materials to reduce the risk of explosion and fire while improving performance.
 (From the bottom left) Professor Donghwa Seo of KAIST, Researcher Jaeseung Kim of KAIST, (from the top left) Professor Kyungwan Nam of Dongguk University, Professor Sungkyun Jung of Seoul National University, Professor Yunseok Jung of Yonsei University. Courtesy of KAIST
(From the bottom left) Professor Donghwa Seo of KAIST, Researcher Jaeseung Kim of KAIST, (from the top left) Professor Kyungwan Nam of Dongguk University, Professor Sungkyun Jung of Seoul National University, Professor Yunseok Jung of Yonsei University. Courtesy of KAIST
In conventional batteries, lithium ions move through a liquid electrolyte. In contrast, all-solid-state batteries use a solid electrolyte instead of a liquid, making them relatively safer. However, enabling lithium ions to move quickly within a solid requires the use of expensive metals or complex manufacturing processes, which has been a limiting factor.
To overcome these limitations, the joint research team focused on divalent anions such as oxygen and sulfur. They created pathways within the all-solid-state electrolyte that allow lithium ions to move smoothly. Divalent anions play a role in altering the crystal structure by becoming part of the basic framework of the electrolyte’s internal structure.
The core of the technology developed by the joint research team is the precise control of the internal structure of low-cost zirconium (Zr)-based halide all-solid-state electrolytes by introducing divalent anions. This design principle, called the "framework tuning mechanism," widens the pathways for lithium ion movement inside the electrolyte and lowers the barriers encountered during migration. By adjusting the bonding environment and crystal structure around lithium ions, the ions can move faster and more easily.
To verify these structural changes, the joint research team used advanced analytical techniques such as ultra-high-resolution X-ray scattering analysis, pair distribution function (PDF) analysis, X-ray absorption spectroscopy (XAS), and computer-based electronic structure and diffusion modeling (DFT) to identify changes at the atomic level.
During this analysis, it was found that electrolytes incorporating oxygen or sulfur exhibited lithium ion mobility that was more than two to four times higher than that of conventional zirconium-based electrolytes. This demonstrates that it is possible to achieve performance suitable for practical application in all-solid-state batteries even when using inexpensive materials.
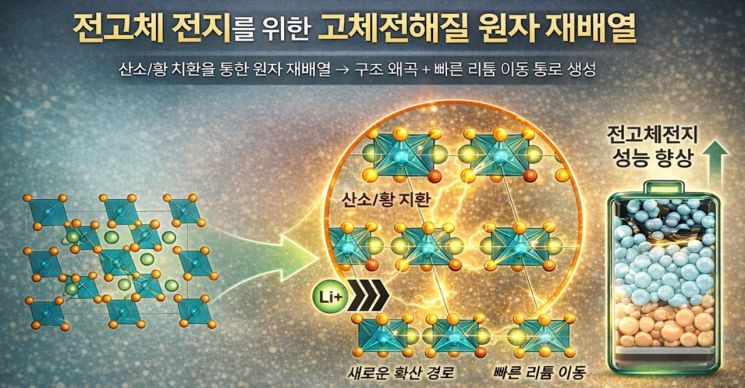 Atomic rearrangement process of solid electrolytes for all-solid-state batteries (AI-generated image). Provided by KAIST
Atomic rearrangement process of solid electrolytes for all-solid-state batteries (AI-generated image). Provided by KAIST
In fact, the room-temperature ionic conductivity of the oxygen-doped electrolyte was measured at 1.78 mS/cm, while that of the sulfur-doped electrolyte was 1.01 mS/cm. Ionic conductivity is an indicator of how quickly and efficiently lithium ions can move within the electrolyte; the higher the value, the better the battery performance. A value above 1 mS/cm is considered sufficient for practical application in batteries at room temperature.
Professor Seo stated, "This research is significant because it presents a design principle that can simultaneously address the cost and performance issues of all-solid-state batteries using inexpensive raw materials."
Meanwhile, this study was co-led by Researcher Jaeseung Kim of KAIST and Researcher Dasul Han of Dongguk University as joint first authors. The results were recently published in the international journal Nature Communications.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















