First in Asia...
Technical Support Provided Through the National Containment Certification Committee
The Korea Disease Control and Prevention Agency announced on October 21 that LG Chem's polio vaccine production facility has become the first in Asia and the third in the world to receive containment certification from the World Health Organization (WHO).
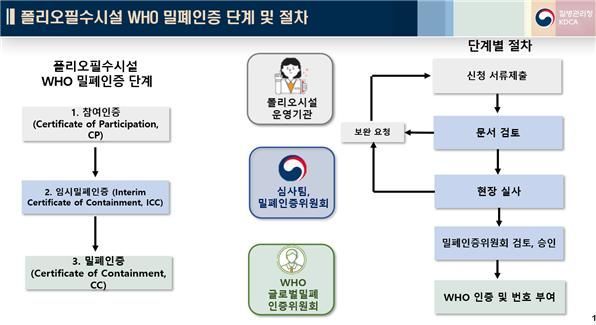
The polio vaccine production facility handles poliovirus, which causes poliomyelitis, to manufacture polio vaccines. Countries are required to destroy unnecessary poliovirus, and if they retain the virus, they must undergo government review and obtain WHO certification as a "polio essential facility" to prevent any potential leaks. Since 1988, WHO has established the Global Polio Eradication Initiative (GPEI) and has required extensive national vaccination campaigns for polio eradication. WHO also mandates that polio essential facilities obtain containment certification by 2026.
LG Chem established an injectable polio vaccine production line in 2018, received WHO prequalification (PQ) approval in 2020, and has been supplying vaccines to the United Nations Children's Fund (UNICEF) and other organizations since 2021. Currently, only three countries-South Korea, Belgium, and Denmark-have facilities that have obtained WHO containment certification for polio essential facilities.
The acquisition of WHO containment certification for domestic polio essential facilities is significant, as it not only demonstrates the safety of polio vaccine production facilities but also internationally recognizes the country's biosafety management capabilities. This means that the facility is equipped with the safety and risk management capabilities required for handling poliovirus and can be utilized for the development and production of vaccines for emerging infectious diseases with high risk potential in the future.
WHO containment certification is a process that evaluates the standards for containment facilities and risk management systems for the safe handling of poliovirus. It is based on the WHO guideline "Global Action Plan, 4th Edition (GAPIV)," and certification for polio essential facilities is granted only when all 14 detailed criteria are met, covering all areas of biosafety and biosecurity, including biosafety management systems, education and training, security, physical containment, and emergency response plans.
Since 2018, the Korea Disease Control and Prevention Agency has established a National Containment Certification Committee, provided risk management education and on-site inspections for polio essential facilities, participated in WHO-led polio response training, and continued to offer technical support and cooperation with WHO for containment certification.
Im Seungkwan, Commissioner of the Korea Disease Control and Prevention Agency, stated, "With the acquisition of WHO polio containment certification, our country's advanced national biosafety management system and the risk management capabilities of domestic polio vaccine manufacturers have gained international recognition." He added, "We will conduct additional national surveys on polio infectious materials in accordance with the WHO Global Polio Eradication Initiative and actively participate in international efforts to eradicate polio."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.