171 IND Approvals Last Year
12% Increase Compared to Previous Year
Domestic biotech companies are increasingly entering the development of 'targeted anticancer drugs,' which reduce side effects while enhancing therapeutic efficacy. With the number of clinical trial applications (IND) for targeted anticancer drugs recording double-digit growth last year, these companies are accelerating their efforts to penetrate the global anticancer drug market, which is expected to grow to an annual scale of 700 trillion won.
According to the "2024 Korea Clinical Trial Industry Information Statistics Book" published by the Korea National Enterprise for Clinical Trials on October 13, the number of IND approvals for targeted anticancer drugs last year reached 171, an increase of about 12% from 153 in the previous year. IND approval is the process by which regulatory authorities grant permission to conduct clinical trials in order to verify the safety and efficacy of a new drug candidate under development.
Targeted anticancer drugs selectively attack specific genes or proteins that are essential for the growth and survival of cancer cells. They are regarded as next-generation anticancer drugs that improve upon the side effects of conventional cytotoxic chemotherapy, such as hair loss, vomiting, and leukopenia caused by damage to normal cells.
The increase in IND approvals for targeted anticancer drugs appears to be driven by the success stories of domestically developed new drugs and the rapid growth of the market. A leading example among domestic companies is "Lecraza" (ingredient: lazertinib) by Yuhan Corporation. Lecraza, when used in combination with Janssen's "Ryvrevant," serves as a first-line treatment for patients with metastatic non-small cell lung cancer who have specific genetic mutations. In the first half of this year, global sales of the Lecraza and Ryvrevant combination therapy reached approximately 320 million dollars (about 448 billion won).
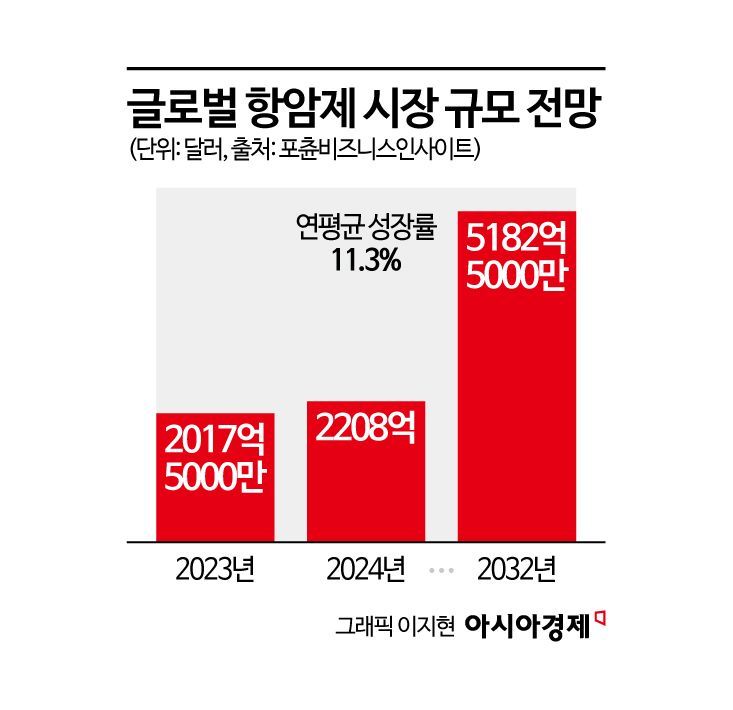
The anticancer drug market itself is also growing rapidly. According to market research firm Fortune Business Insights, the global anticancer drug market, which was valued at 201.75 billion dollars (about 282.6518 trillion won) in 2023, is projected to reach 518.25 billion dollars (about 726.0683 trillion won) by 2032, with an average annual growth rate of 11.3%.
Meanwhile, domestic companies aiming to develop a "second Lecraza" are also speeding up their efforts. Hanmi Pharmaceutical is developing "SOS1-KRAS Interaction Inhibitor (HM101207)" as a targeted anticancer drug candidate. This drug targets the "KRAS mutation," known as one of the most lethal cancer-causing genes. HM101207 is a new mechanism therapy that blocks the binding of the "SOS1" protein, which is essential for KRAS protein activation.
J INTS BIO is conducting Phase 1/2 clinical trials of "JIN-A02," a fourth-generation EGFR (epidermal growth factor receptor) targeted anticancer drug, in Korea, the United States, and Thailand. According to J INTS BIO, patients who developed resistance to existing treatments maintained their response to the drug for over one year and ten months. Notably, among three patients with brain metastases, one showed complete disappearance of brain lesions.
Lee Seungkyu, Vice Chairman of the Korea Biotechnology Industry Organization, said, "Among various new drug fields, the market outlook for targeted anticancer drugs is particularly promising," adding, "As part of a strategy of selection and concentration, interest is increasingly focused on the development of targeted anticancer drugs."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















