Third Order Secured This Year
Focus on Syracuse Plant in New York
Positive Outlook After Songdo Plant Completion
Even before completing its plant in Songdo, Incheon, Lotte Biologics is securing a series of orders from global pharmaceutical companies through its U.S. facility. The company expects that these achievements will also boost orders for its domestic plant, which will operate under the same quality standards.
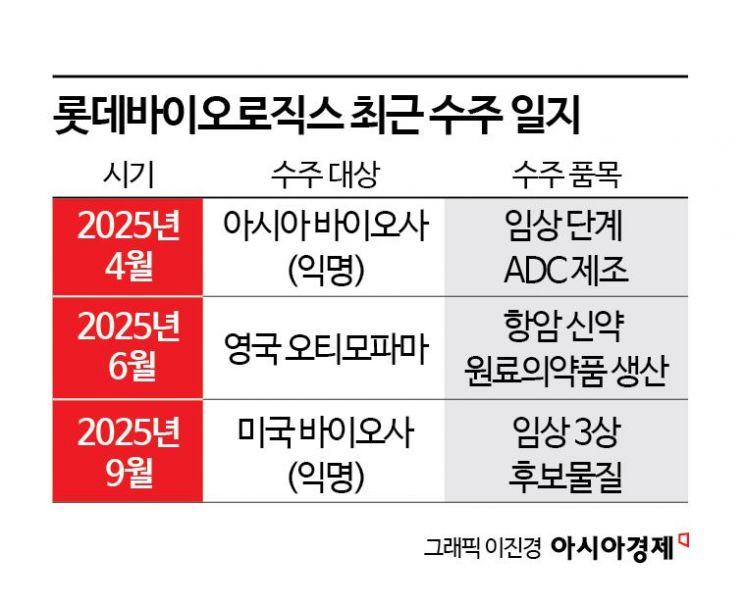
According to the industry on September 16, Lotte Biologics recently signed a contract for biopharmaceutical contract manufacturing (CMO) with a global bio company headquartered in the United States. This marks the third order this year, following an April contract with an Asia-based company for the production of clinical trial candidate materials for an antibody-drug conjugate (ADC), and a June contract with Otimo Pharma for antibody drug CMO services.
All three orders signed this year will be produced at the company’s Syracuse plant in New York State. Lotte Biologics acquired the Syracuse manufacturing facility from Bristol Myers Squibb (BMS) in 2022. Building on this, the company has continued to secure contract manufacturing agreements with U.S. pharmaceutical companies, establishing trust in the North American market. The Syracuse plant is an FDA-approved facility and is advantageously located near the pharmaceutical cluster on the U.S. East Coast. The ADC manufacturing facility, which received an investment of about 100 million dollars (approximately 140 billion won), can provide 'one-stop services' including production, purification, quality control, and characterization, based on its cGMP (current Good Manufacturing Practice) competitiveness.
These order references are expected to translate into increased trust for the Songdo plant. When commercial production at the Songdo facility begins in 2027, Lotte Biologics will fully implement its 'dual base' strategy connecting the United States and Korea. The company is pushing forward with the construction of the Songdo Bio Campus, which will consist of three manufacturing facilities, each with a production capacity of 120,000 liters. The first plant, dedicated to antibody drug production, is scheduled for completion in 2026, with the goal of commencing commercial production in the first half of 2027. Upon completion of the first plant, the company will secure a total production capacity of 160,000 liters, including the 40,000-liter capacity of the Syracuse Bio Campus in the United States.
The Syracuse plant offers advantages in initial volumes and rapid response due to its physical proximity to clients, while the Songdo plant provides economies of scale and cost competitiveness through its large-scale facilities. This dual-base strategy supports the 'supply chain stability' that global pharmaceutical companies value most. Even if a problem occurs at one site, supply can continue from the other production base, making risk diversification easier. At the same time, clients benefit from receiving production services that meet the same quality standards in both the United States and Asia.
The recent reestablishment of the Donald Trump administration in the United States and its strengthened 'onshoring' (bringing manufacturing back to the U.S.) policy further validates Lotte Biologics' strategic decisions. Only companies with production bases in the United States can secure advantages in terms of tax benefits and regulatory compliance. By securing the Syracuse plant early on, Lotte Biologics has positioned itself to respond quickly to policy changes. An industry insider commented, "For global pharmaceutical companies, partnering with a company that has U.S.-based manufacturing capacity, in line with the onshoring trend, is a way to reduce risks. At the same time, large-scale, low-cost production at the Songdo base in Asia ensures cost efficiency."
Support from Lotte Group also provides a strong foundation. At the recent topping-out ceremony for the Songdo plant, Shin Yooyul, Head of Global Strategy at Lotte Biologics and Head of Future Growth at Lotte Holdings, attended in person, demonstrating the group's commitment. Shin emphasized, "Lotte Biologics will grow into a new growth engine for the group and a company representing the future," pledging full support for the Songdo project and global expansion.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.