KRW 14.75 Billion in Funding Over 4.5 Years
Seoul National University Hospital announced on September 10 that it has been selected as the lead institution for the 2025 Korean ARPA-H Project research initiative and will be undertaking the "Development of a Personalized Innovative Treatment Platform for Pediatric Rare Diseases and N-of-1 Clinical Trials."
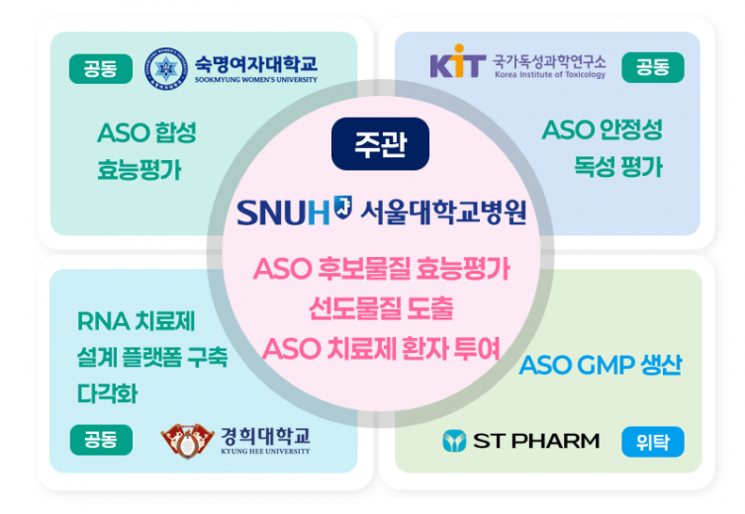
This project is a research and development initiative supported by the Ministry of Health and Welfare, the Korea Health Industry Development Institute, and the K-Health Future Promotion Group. It will provide up to 14.75 billion KRW in funding over four years and six months. Seoul National University Hospital will form a consortium with Sookmyung Women's University, the National Institute of Toxicological Research, Kyung Hee University, and STpharm Co., Ltd., and will conduct joint research to present a new paradigm for the treatment of pediatric rare diseases in Korea.
There are over 7,000 types of rare diseases, and 80% of them manifest during childhood. While advancements in genomic medicine and institutional support have gradually increased the diagnostic rate, most of these diseases still lack suitable treatments even after diagnosis. In particular, for life-threatening severe pediatric rare diseases, the traditional drug development process, which takes an average of more than 10 years, makes it difficult to secure treatment opportunities. As a result, strategies to design drugs based on patients' genetic mutations are being pursued worldwide to accelerate new drug development.
The Seoul National University Hospital consortium will utilize antisense oligonucleotide (ASO) technology to begin designing and producing personalized therapeutics for rare disease patients. This technology works by binding to the mRNA of specific genes to regulate protein expression and correct the fundamental cause of the disease. It is one of the most actively researched fields in precision medicine-based therapeutics. Furthermore, the consortium plans to develop and administer an ASO therapeutic tailored for a single patient, conducting the nation's first "N-of-1 clinical trial."
This research is significant in that it aims to establish a new treatment platform for rare diseases through the development and clinical trials of personalized gene therapies for patients with severe pediatric rare diseases. Additionally, it is designed to be applicable to patients with other genetic abnormalities, thereby increasing treatment accessibility for rare disease patients in Korea and providing world-class, precision medicine-based innovative therapies.
Chae Jonghee, professor at the Department of Clinical Genomic Medicine at Seoul National University Hospital and principal investigator, stated, "This research will be an important turning point that can offer new hope to pediatric patients suffering from rare diseases and their families," adding, "We will do our utmost to ensure that patient-customized gene therapies can be applied to actual patients through this project."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.