UNIST, KAIST, and Stanford University Develop Self-Driven Hydrogen Peroxide Production System Based on Glycerol Oxidation
Simultaneous Hydrogen Peroxide Generation, Power Production, and High-Value Compound Conversion... Published in Nat. Synth.
An international joint research team from UNIST, KAIST, and Stanford University has developed a technology that can produce green hydrogen peroxide (H₂O₂) without external electricity or solar energy for the first time.
On September 10, Professor Jiwook Jang’s team from the Department of Energy and Chemical Engineering at UNIST announced that, together with Professor Donghwa Seo of KAIST and Professor Thomas Haramiya’s team at Stanford University, they have developed a system that produces green hydrogen peroxide using glycerol, a byproduct of biodiesel, without the need for electricity or solar energy.
This system not only operates without external power but can even generate electricity, and it also produces high-value-added glyceric acid simultaneously.
 Research team, (from left) Professor Jiwook Jang of UNIST, Professor Donghwa Seo of KAIST, Professor Thomas Haramiya of Stanford University, Researcher Dongrak Oh, Researcher Sunwoo Hwang, Researcher Dongyeon Kim, Researcher Matthew Jesse. Provided by UNIST
Research team, (from left) Professor Jiwook Jang of UNIST, Professor Donghwa Seo of KAIST, Professor Thomas Haramiya of Stanford University, Researcher Dongrak Oh, Researcher Sunwoo Hwang, Researcher Dongyeon Kim, Researcher Matthew Jesse. Provided by UNIST
Hydrogen peroxide, widely known as a disinfectant, is an industrial raw material with over 90% of its total production consumed in processes such as pulp bleaching and semiconductor cleaning. Although demand is expected to rise due to its potential as a fuel cell oxidant and energy storage medium, current production relies on the anthraquinone process, which uses expensive hydrogen, organic solvents, and large amounts of fossil fuels. This process also generates significant organic pollutants and carbon dioxide emissions.
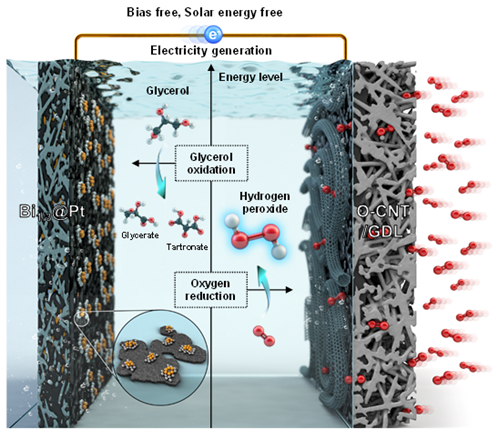
The production system developed by the research team produces hydrogen peroxide without pollutants or carbon dioxide emissions, and does not require external energy sources such as electricity or solar power. This is achieved by utilizing the chemical energy of glycerol. At the anode, glycerol spontaneously oxidizes into glyceric acid, releasing electrons; these electrons travel to the cathode, where they reduce oxygen to produce hydrogen peroxide (H₂O₂). Electricity is generated during this transfer process.
While the principle is similar to that of a battery, where zinc is oxidized and manganese dioxide is reduced to generate electricity, unlike batteries where zinc and manganese dioxide are consumed, this system produces hydrogen peroxide and glyceric acid.
The research team designed the system so that glycerol oxidation and oxygen reduction reactions occur at the maximum theoretically possible potential difference, by applying a platinum catalyst coated with bismuth at the anode and carbon nanotubes at the cathode. The larger the potential difference, the greater the 'energy drop' available for electrons to flow, making both reactions easier to occur.
Experimental results showed that the system produced approximately 8,475 micromoles (μmol) of hydrogen peroxide per square centimeter per minute. This is comparable to the production rate per unit area of the anthraquinone process.
Additionally, the reaction converting glycerol to glyceric acid achieved a high reaction selectivity of 74%. Reaction selectivity indicates the proportion of the desired product, rather than byproducts, formed from the reactants; the higher the value, the purer the glyceric acid produced. Glyceric acid is used in pharmaceuticals, cosmetics, and biodegradable polymer materials, and is estimated to have about 3,000 times higher economic value than glycerol.
Professor Jiwook Jang stated, "Previous eco-friendly production technologies still relied on fossil fuel-based electricity or required solar power, which imposed economic limitations such as the need for land and operational time. This technology overcomes those constraints." He added, "By using glycerol, a low-cost biodiesel byproduct, we can simultaneously produce hydrogen peroxide and high-value compounds, and even recover electricity, making this system both economically viable and sustainable."
This research was jointly led by Dr. Dongrak Oh of UNIST (currently a postdoctoral researcher at KIST), Researcher Sunwoo Hwang of UNIST, Researcher Dongyeon Kim of KAIST (currently a professor at Jeonbuk National University), and Researcher Jesse Matthews of Stanford University as co-first authors.
The results were published in the August issue of 'Nature Synthesis,' a prestigious journal in the field of chemical synthesis research.
The research was supported by the Brainlink Program for Excellent Researcher Exchange, the Global Research Laboratory (BRL) Program, and the Mid-Career Researcher Program of the National Research Foundation of Korea under the Ministry of Science and ICT.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















