First-Ever Observation of Structural Intermediates in Membrane Protein Pairing
Discovery by Professor Min Duyeong's UNIST Team
Paves the Way for New Drug Development Targeting Membrane Protein Interactions
Published in Nature Communications
The "hidden steps" in the process by which cell membrane proteins form pairs have been captured for the first time in the world.
Contrary to the conventional belief that proteins bind all at once, it has now been confirmed at the level of a single protein molecule that binding is actually completed through several intermediate steps, much like a zipper.
The research team led by Professor Min Duyeong of the Department of Chemistry at Ulsan National Institute of Science and Technology (UNIST) announced on September 8 that they have tracked the process by which cell membrane proteins pair up in real time and have identified the intermediate stages involved.
 Research team, (from left) Professor Dooyoung Min, Researcher Seoyoon Kim (first author), Researcher Sadongo Victor (first author), Researcher Eojin Kim (first author), Researcher Wijesinghe Bashini. Provided by Ulsan National Institute of Science and Technology (UNIST)
Research team, (from left) Professor Dooyoung Min, Researcher Seoyoon Kim (first author), Researcher Sadongo Victor (first author), Researcher Eojin Kim (first author), Researcher Wijesinghe Bashini. Provided by Ulsan National Institute of Science and Technology (UNIST)
The membrane surrounding a cell contains numerous embedded proteins. These membrane proteins serve as gateways, receiving external signals or transmitting signaling molecules. More than 50% of these proteins must form pairs to function properly.
This study revealed that, during this process, membrane proteins bind to each other gradually. Rather than attaching immediately, the proteins interlock at specific sites and pass through multiple intermediate stages before finally forming a complete pair. Previously, it was believed that two membrane proteins would approach and bind all at once.
The research team was able to uncover this through a new analytical method called "single-molecule tweezers for membrane protein interactions." This technique uses a tweezer-like tool to grasp both proteins and pull them apart, recording in real time how the binding proceeds and breaks.
This conclusion was further supported by additional experiments. The researchers inserted a short peptide fragment between the membrane proteins to disrupt their binding. As a result, the binding stopped at an intermediate stage. Just as a zipper cannot close completely if a tooth in the middle is broken, membrane proteins cannot complete their binding if a specific stage is blocked.
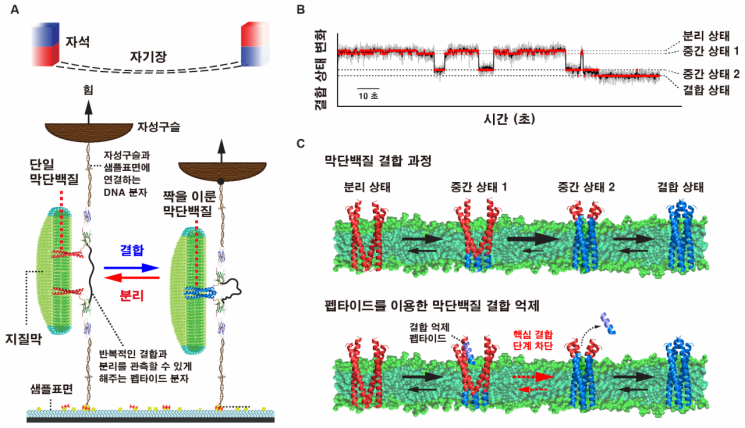 The research team revealed the binding process of membrane proteins using single-molecule tweezer technology.
The research team revealed the binding process of membrane proteins using single-molecule tweezer technology.
Professor Min Duyeong stated, "The discovery that membrane proteins bind sequentially through intermediate stages marks a major turning point in our understanding of protein-protein interactions. The principle of inhibiting membrane protein binding is already applied in the breast cancer drug Perjeta. By revealing these hidden steps in the binding process and selectively blocking them, it will be possible to design more effective new drugs."
Professor Min added, "The single-molecule tweezer analysis method used in this study can be applied to precisely investigate membrane protein binding processes that are important in medicine and pharmacology."
The research results were published in the prestigious journal Nature Communications on August 9.
This research was supported by the National Research Foundation of Korea and Ulsan National Institute of Science and Technology (UNIST).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















