U.S. FDA Launches 'PreCheck Program'
to Accelerate Construction of Pharmaceutical Manufacturing Facilities
As U.S. President Donald Trump signaled the imposition of high tariffs on pharmaceuticals, the U.S. Food and Drug Administration (FDA) has introduced the 'PreCheck Program' aimed at drastically reducing the construction period for domestic pharmaceutical manufacturing facilities. This move is interpreted as a clear indication that the administration is now formally incorporating the biomanufacturing sector as a core industry, following semiconductors, batteries, and automobiles.
According to the Bioeconomy Research Center of the Korea Bio Association on August 11, the FDA officially announced the 'FDA PreCheck Program' on August 7 (local time), stating its intention to support the Trump administration’s policy of reducing dependence on foreign pharmaceuticals and strengthening the domestic supply chain. This initiative is an extension of the 'Executive Order on Promoting Domestic Production of Essential Medicines' signed in May, and its core objective is to significantly shorten the construction period for pharmaceutical manufacturing facilities, which has traditionally taken five to ten years or more.
Currently, more than half of the pharmaceuticals distributed in the United States are manufactured overseas. In particular, only 11% of active pharmaceutical ingredients (APIs), the key raw materials for drugs, are produced domestically. In response, the FDA has established an unprecedented fast-track system through pre-approvals and early communication to bring manufacturing infrastructure back to the U.S.
The PreCheck Program consists of two main stages. The first is the facility preparation stage, during which the FDA works closely with companies throughout design, construction, and pre-production phases to review approval requirements in advance. Companies may submit their operational plans, quality systems, and management maturity assessments ahead of time, and these documents can be officially referenced during the final approval application. The second stage involves the application submission, where companies engage in pre-meetings and feedback sessions with the FDA before formally applying for approval, thereby streamlining the documentation process for Chemistry, Manufacturing, and Controls (CMC).
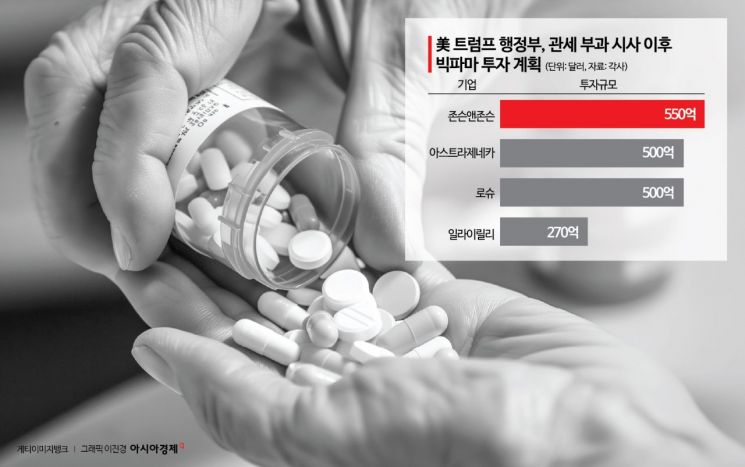
This approach is seen as the U.S. administration’s strategy to accelerate the establishment of domestic manufacturing facilities by simplifying administrative procedures. In fact, following the Trump administration’s announcement of potential tariffs, global pharmaceutical companies such as Johnson & Johnson ($55 billion, approximately 76.53 trillion KRW), AstraZeneca and Roche (each $50 billion, approximately 69.58 trillion KRW), and Eli Lilly ($27 billion, approximately 37.57 trillion KRW) have recently announced large-scale investments in U.S. plant construction. Generic drug companies such as Aurobindo from India and Hikma from the UK, as well as South Korea’s Celltrion, are also seeking to expand their presence in the U.S. market.
Particularly, the fact that a 15% tariff on European pharmaceuticals is expected to be imposed as early as this month further highlights the effectiveness of the FDA PreCheck Program. Not only will tariffs be applied according to the U.S.-EU trade agreement, but depending on the results of the ongoing 'Section 232' investigation, pharmaceuticals could be designated as national security assets, leading to the implementation of additional non-tariff barriers and other sanctions.
An industry insider commented, "Biopharmaceutical manufacturing facilities are now included in the broader strategy of attracting advanced manufacturing facilities to the U.S., alongside traditional sectors such as semiconductors, batteries, and electric vehicles. The pharmaceutical industry, which has historically seen a strong trend of 'offshoring' due to lengthy regulatory and approval timelines, is now shifting to become a central pillar of the 'reshoring' policy focused on domestic production."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.