On July 31, global healthcare company Moa Life Plus announced that it has successfully completed animal efficacy tests on its proprietary functional ingredient, poly-gamma-glutamic acid potassium (PGA-K), for its ability to protect the gastric mucosa from gastric acid and suppress alcohol-induced gastric injury. Based on these results, the company has filed for a patent.
This experiment was conducted in collaboration with the Gochang Food Industry Research Institute, after being selected for the Ministry of Trade, Industry and Energy's 'Agro-Bio Material-Based Industrialization Technology Promotion Project.' The study scientifically confirmed in animal models the multifaceted gastric protective effects of PGA-K, including protection and inhibition of damage to the gastric mucosa, reduction of inflammatory responses, and regulation of excessive gastric acid secretion.
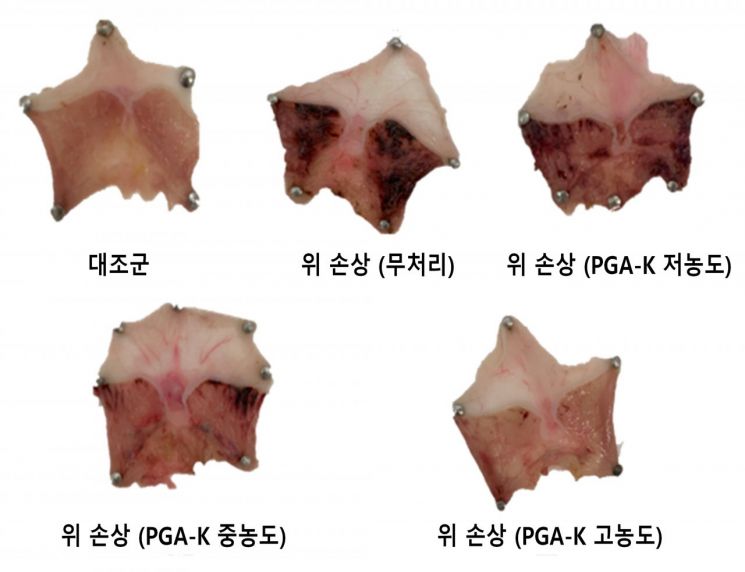
In particular, when mice were administered PGA-K daily for two weeks and then subjected to alcohol-induced gastric injury, the group treated with PGA-K showed a significantly lower gastric mucosal damage index compared to the control group that did not receive PGA-K. The expression levels of inflammation-inducing cytokines in gastric tissue were also markedly lower. This indicates that PGA-K is not merely a gastric mucosal protectant but is a physiologically active functional substance that can contribute to improving gastric function.
PGA-K is a novel material derived from natural polymers found in traditional fermented foods, re-developed using modern liquid fermentation and pure refining technology. It is currently used as a raw material for health functional foods and cosmetics, focusing on functionalities such as immune enhancement, promotion of calcium absorption, and moisturizing effects. The demonstration of its gastric health improvement functionality is expected to serve as an important turning point, highlighting the potential for new functional applications of PGA-K.
A Moa Life Plus representative stated, "Based on the results of the gastric health efficacy tests, we have filed for a patent. Following the individual recognition of PGA-K as a functional ingredient for immune function enhancement, we are also preparing follow-up studies, including human application trials, to obtain individual recognition as a functional ingredient for gastric health improvement." The representative added, "We are also pursuing patents related to improving intestinal health through enhanced bowel movements, so the multifunctionality of PGA-K will attract even more attention in the health functional food market focused on individually recognized ingredients."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














