UNIST and KITECH Develop Denitrification Catalyst with Significantly Expanded Operating Temperature Range
Removes Sources of Fine Dust and Ozone Pollution... Published in Appl. Catal. B: Environ.
Nitrogen oxides are air pollutants emitted wherever fuel is burned, such as in factory smokestacks, automobiles, and ships.
The temperature of nitrogen oxides released varies depending on the type of fuel and equipment operating conditions. A new catalyst has been developed that can consistently remove nitrogen oxides despite these changes.
A research team led by Professor Seungho Cho from the Department of Materials Science and Engineering at UNIST, in collaboration with Dr. Hongdae Kim's team from the Ulsan Technology Commercialization Center at the Korea Institute of Industrial Technology, has developed a denitrification catalyst capable of removing nitrogen oxides (NOx) over a wide temperature range of 240 to 400°C.
 Research team, Professor Seungho Cho (left) and Dr. Myungjin Lee. Provided by UNIST
Research team, Professor Seungho Cho (left) and Dr. Myungjin Lee. Provided by UNIST
Nitrogen oxides released into the atmosphere cause fine dust, ozone pollution, and acid rain. Although selective catalytic reduction (SCR) is used to convert nitrogen oxides into harmless nitrogen, commercial vanadium-tungsten catalysts achieve high efficiency mainly at 350°C. As a result, performance drops sharply in real-world settings where temperature fluctuations are significant.
In contrast, the catalyst developed by the research team achieves a nitrogen oxide removal efficiency of 93.6% at 240°C and maintains a stable conversion efficiency of over 97% even at higher temperatures. Commercial SCR catalysts only reach about 62.4% efficiency at 240°C.
Additionally, more than 97% of the nitrogen oxides were converted into nitrogen (N₂), and there was almost no formation of byproducts from side reactions, such as nitrous oxide (N₂O), a greenhouse gas. The catalyst's lifespan was also improved.
The high performance of the catalyst is attributed to the addition of a small amount of hexagonal boron nitride (h-BN) to the commercial catalyst. Hexagonal boron nitride (h-BN) keeps the vanadium metal ions in the catalyst in an active state and protects the catalyst surface from contaminants such as sulfates or moisture. If contaminants adhere to the surface, the catalyst's lifespan is reduced.
The research team also verified the catalyst's performance for commercialization by agglomerating the powder catalyst into a lump form used in actual industrial settings. While powder catalysts are most reactive, they cannot be used in factories due to issues such as dust and pressure loss.
This catalyst, agglomerated into a honeycomb structure (monolith), demonstrated stable performance in processing tens of micrograms of NO per second under fast gas flow conditions of 20 L/min.
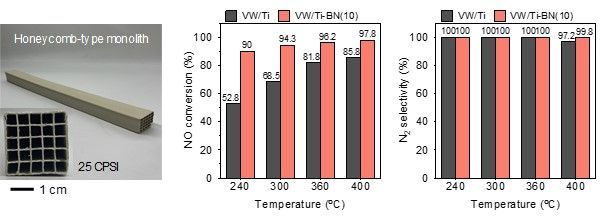 Performance evaluation results of honeycomb-shaped catalysts made by agglomerating powder-form catalysts at high temperatures.
Performance evaluation results of honeycomb-shaped catalysts made by agglomerating powder-form catalysts at high temperatures.
Professor Seungho Cho stated, "This catalyst has a wide operating temperature range, allowing it to reliably remove nitrogen oxides, which are air pollutants emitted from various sources such as factories, automobiles, and ships. By reducing the vanadium content, which is both toxic and expensive, we expect to simultaneously enhance the safety and cost-effectiveness of industrial environments."
This research was published online on June 12 in 'Applied Catalysis B: Environmental and Energy,' an international journal with the highest impact factor (IF: 21.1) in the field of environmental engineering.
Graduate Myungjin Lee participated as the first author.
This research was supported by the National Research Foundation of Korea under the Ministry of Science and ICT, the Korea Institute for Advancement of Technology under the Ministry of Trade, Industry and Energy, and the Korea Institute of Industrial Technology.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














