A next-generation therapeutic agent that selectively targets Candida species to enhance treatment efficacy while reducing side effects has been developed in South Korea.
Candidiasis is an infectious disease caused by Candida, a type of fungus (fungi), which spreads throughout the body via the bloodstream, leading to organ damage and sepsis. In recent years, the incidence of candidiasis has been rising, in line with increases in immunosuppressive therapies, organ transplants, and the use of medical devices.
 (From left) KAIST integrated MS-PhD program student Jooyeon Jung, Professor Hyunjung Jung, integrated MS-PhD program student Seungjoo Yang, integrated MS-PhD program student Ahyoung Park, and other members of the joint research team. Provided by KAIST
(From left) KAIST integrated MS-PhD program student Jooyeon Jung, Professor Hyunjung Jung, integrated MS-PhD program student Seungjoo Yang, integrated MS-PhD program student Ahyoung Park, and other members of the joint research team. Provided by KAIST
On July 8, KAIST announced that the research team led by Professor Hyunjung Jung from the Department of Biological Sciences, in collaboration with Professor Yongpil Jung's team at Asan Medical Center, has developed a "gene-based nano-therapeutic (FTNx)" that simultaneously inhibits two key enzymes in the Candida cell wall.
Currently available antifungal agents for Candida have low target selectivity and may also affect human cells. As a result, new drug-resistant strains have emerged, leading to a gradual decline in treatment efficacy.
In particular, patients with compromised immune systems experience rapid progression of infection and poor prognosis, making it difficult to restore health with existing treatments. This underscores the need for new approaches to treating candidiasis.
The therapeutic agent developed by the joint research team can be administered systemically. By combining gene suppression technology with nanomaterial technology, the team effectively overcame the structural limitations of conventional compound-based drugs. Furthermore, the treatment acts selectively on Candida species, successfully enhancing therapeutic efficacy.
Before developing the therapeutic agent, the researchers created a gold nanoparticle-based complex loaded with short DNA fragments (antisense oligonucleotide·ASO) that simultaneously target β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3), both of which are essential for forming the cell wall of Candida fungi.
Additionally, the team applied a surface-coating technology that binds to a specific glycolipid structure (a structure combining sugar and lipid) in the Candida cell wall, equipping the complex with a targeting device. This ensures that the agent is not delivered to human cells at all, but acts selectively only on Candida, achieving precise targeting.
Once inside the Candida cell, the complex cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of β-1,3-glucan and chitin, which are components of the cell wall. This prevents the Candida cell wall from maintaining structural stability, causing it to collapse and suppressing the survival and proliferation of the fungus.
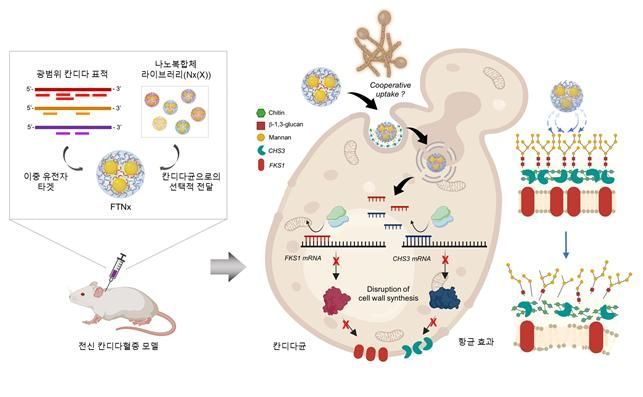 A schematic diagram illustrating the mechanism of action and therapeutic application results of the gene therapy FTNx targeting pathogenic Candida species. Provided by KAIST
A schematic diagram illustrating the mechanism of action and therapeutic application results of the gene therapy FTNx targeting pathogenic Candida species. Provided by KAIST
The joint research team validated the therapeutic efficacy using a systemic candidiasis model in laboratory mice, observing a reduction in fungal burden within organs, normalization of immune responses, and a significant increase in survival rate in the treatment group.
Professor Hyunjung Jung stated, "Through this study, the joint research team has not only proposed a way to overcome the issues of human toxicity and the spread of drug resistance associated with existing treatments but also confirmed the potential of gene therapy for systemic infections. We plan to continue optimizing the administration method and conducting toxicity validation studies for future clinical applications."
This research, supported by the Ministry of Health and Welfare and the National Research Foundation of Korea, was conducted with Jooyeon Jung from KAIST's Department of Biological Sciences and Yoonkyung Hong from Asan Medical Center as co-first authors. The research paper was published on July 1 in the international journal Nature Communications.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)















