Enhancing Methanol Production from Carbon Dioxide with a Copper-Based Composite Catalyst
Achieving Noble Metal-Level Performance and Innovative Structure Design Using Battery Discharge Principles, Published in Advanced Materials
A domestic research team has developed a technology to convert carbon dioxide (CO₂) into methanol.
This technology is attracting attention as it can reduce CO₂, a major cause of global warming, while also producing eco-friendly fuel.
On June 26, a team led by Professor Jeongki Ryu from the Department of Energy and Chemical Engineering at UNIST announced that, in collaboration with Professor Jongsun Kim's team at Sungkyunkwan University and Professor Aloysius Son's team at Yonsei University, they have developed a copper catalyst that converts carbon dioxide into methanol.
 Research team, (from left) Professor Jeongki Ryu, Dr. Hyunwoo Kim (first author), Researcher Suhwan Park. Provided by UNIST
Research team, (from left) Professor Jeongki Ryu, Dr. Hyunwoo Kim (first author), Researcher Suhwan Park. Provided by UNIST
Methanol is a basic chemical feedstock for products such as plastics and synthetic fibers. It is also gaining attention as an energy source because it can be easily stored and transported in liquid form, and is used as a hydrogen carrier and fuel cell material. While producing methanol from carbon dioxide can reduce carbon emissions, the process typically results in the mixing of substances like hydrogen, requiring an additional purification step.
The copper catalyst developed by the research team can selectively produce only methanol, not byproducts. The methanol selectivity, which refers to the ability to selectively produce only the target product, reached up to 70%?the highest among copper-based catalysts and comparable to the performance of expensive noble metal catalysts. In contrast, the selectivity of conventional copper catalysts remains at the 10?30% level.
This catalyst features a closely integrated structure in which copper pyrophosphate (Cu₂P₂O?) nano-domains and pure copper metal domains fit together like puzzle pieces. This structure suppresses competing reactions that produce hydrogen and enables the selective production of methanol.
Generally, it is difficult to create such a sophisticated composite structure, but the research team achieved this easily by applying the discharge principle of lithium batteries. By passing an electric current through the electrode material, as in battery discharge, part of the copper pyrophosphate in the electrode is reduced to pure copper, naturally forming a composite structure in which both substances are mixed within a single particle. Materials other than the catalyst, such as the electrode material, can be easily removed by washing with water.
Another important finding of this research is that the reaction follows a different pathway than previously known methods. Normally, methanol is produced via carbon monoxide (CO), but this catalyst first produces formic acid (HCOOH) and then converts it into methanol. The team explained that this not only provides a basis for developing new methanol synthesis catalysts, but also offers a meaningful clue for broadening the understanding of methanol synthesis pathways.
Professor Jeongki Ryu stated, "Methanol is an important industrial feedstock and energy source, with tens of millions of tons consumed annually worldwide. This catalyst technology, which achieves high selectivity and current density using inexpensive copper, could help usher in an era of 'carbon resource utilization' by enabling the direct conversion of carbon dioxide into valuable resources at industrial sites."
Professor Ryu added, "The fact that we could easily obtain the catalyst by utilizing the battery discharge principle means there is great potential for industrial applications. We plan to expand this technology to actual processes by scaling up the electrode area and integrating it into systems in the future."
This research involved Dr. Hyunwoo Kim and integrated master's and doctoral program researcher Suhwan Park from the Department of Energy and Chemical Engineering at UNIST, integrated master's and doctoral program researcher Jihye Lee from Sungkyunkwan University, and integrated master's and doctoral program researcher Sangseop Lee from Yonsei University.
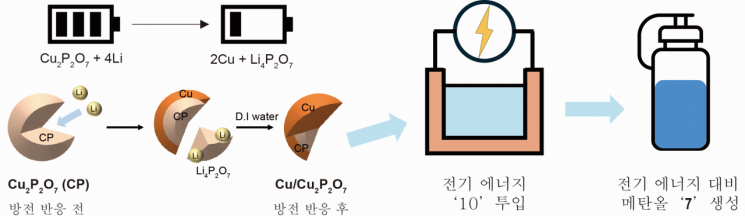 A sophisticated structure of a high-efficiency catalyst was fabricated by applying the battery discharge process.
A sophisticated structure of a high-efficiency catalyst was fabricated by applying the battery discharge process.
In recognition of its excellence and research impact, the study was published online in the world-renowned scientific journal Advanced Materials on May 20, and was supported by the National Research Foundation of Korea under the Ministry of Science and ICT.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










