A technology has been developed that enables the recycling of waste tires into raw materials for rubber and nylon fibers. Every year, billions of waste tires are discarded worldwide, making them one of the main causes of environmental pollution. However, there are expectations that the application of this newly developed technology will mark a turning point in the field of waste tire recycling.
On June 26, KAIST announced that the research team led by Professor Sunhyuk Hong from the Department of Chemistry has opened up the possibility of effectively solving the environmental pollution problem caused by waste tires by developing a dual-catalyst-based continuous reaction system.
 (From left) Kyungmin Choi, integrated master's and doctoral program, Beomsun Park, PhD, Sunhyuk Hong, Professor, Kyeongil Jo, PhD. Provided by KAIST
(From left) Kyungmin Choi, integrated master's and doctoral program, Beomsun Park, PhD, Sunhyuk Hong, Professor, Kyeongil Jo, PhD. Provided by KAIST
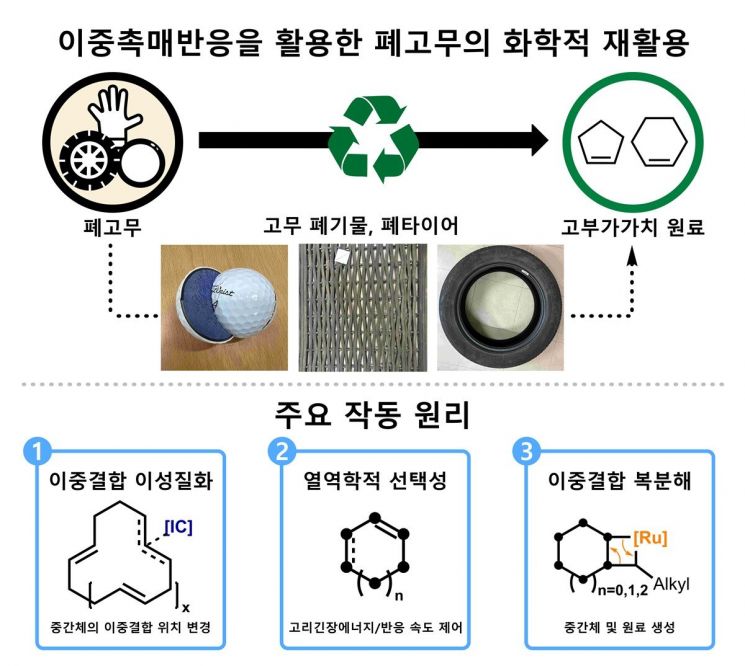
Waste tires are composed of a composite of synthetic and natural rubber. They also contain additives such as silica, carbon black, and antioxidants, which maximize their physical strength and durability. In particular, the vulcanization process forms cross-links between rubber chains, resulting in a structure that is highly resistant to heat and pressure. This is one of the main reasons why chemical recycling of waste tires is so challenging.
Until now, the recycling of waste tires has mainly relied on pyrolysis or physical shredding. The pyrolysis method involves breaking down polymer chains at high temperatures of 350 to 800 degrees Celsius to convert them into fuel oil. However, high energy consumption, low selectivity, and the production of low-quality hydrocarbon mixtures have clearly limited the efficiency of this approach.
To address these issues, the research team developed a method that uses two different catalysts to convert waste rubber into valuable chemicals.
One catalyst alters the bonding structure within the rubber molecules to promote decomposition, while the other catalyst induces a ring-closing reaction to produce cyclic compounds.
This approach achieved selectivity of up to 92% and a yield of 82%. The research team explained that the cyclic pentene produced by the catalyst can be recycled into rubber, and the cyclic hexene can be used as a raw material for nylon fibers, making them highly valuable for industrial applications.
The team successfully applied the developed system to waste tires, converting them into high-purity cyclic alkenes with selective transformation. Unlike conventional pyrolysis, this process enables the production of high-value-added chemical feedstocks through precise catalytic reactions at low temperatures. As such, it is seen as a new turning point in the field of waste tire recycling.
Above all, the research team emphasized that the developed technology can be widely applied to various types of synthetic and waste rubber, making it a key foundational technology that could contribute to the realization of a resource-circulating economy.
Professor Sunhyuk Hong stated, "This research is significant in that it presents an innovative solution for the chemical recycling of waste tires," and added, "The research team plans to lay the groundwork for next-generation high-efficiency catalyst development and commercialization to further enhance the economic feasibility of the technology."
This research was conducted by Beomsun Park, Kyeongil Jo, and Kyungmin Choi, researchers from the Department of Chemistry at KAIST, with support from the National Research Foundation of Korea. The research paper was published online in the international journal 'Chem' on June 18.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















