An inhibitor that prevents the formation of toxic substances responsible for Alzheimer's disease has been developed in South Korea. Alzheimer's disease is the most common and representative type of dementia, and is characterized mainly by the formation of abnormally aggregated clumps (pathogenic fibrillar aggregates) of amyloid beta and tau proteins found in the brains of patients.
 (From left) Professor Jungon Kim, Professor Taesu Choi, Postdoctoral Researcher Dongjun Lim (Research Professor) at Korea University. Provided by the National Research Foundation of Korea
(From left) Professor Jungon Kim, Professor Taesu Choi, Postdoctoral Researcher Dongjun Lim (Research Professor) at Korea University. Provided by the National Research Foundation of Korea
The National Research Foundation of Korea announced on June 4 that a joint research team led by Professors Jungon Kim and Taesu Choi at Korea University, together with Professor William A. Goddard III at the California Institute of Technology, has successfully designed a peptide aggregation inhibitor that suppresses the misfolding and self-aggregation of amyloid beta proteins.
Amyloid beta is the primary protein found in pathogenic fibrillar aggregates in the brains of Alzheimer's disease patients. These pathogenic fibrillar aggregates are formed through the self-aggregation of misfolded amyloid beta proteins and are known to be toxic.
Recently, research to develop therapeutics that fundamentally address the disease by targeting the causative agents of Alzheimer's disease has been active, with an increasing number of successful cases. This clinical success is rooted in the fact that the key target in the development of Alzheimer's disease therapeutics, which have long lacked effective treatments, is the pathogenic fibrillar aggregate of amyloid beta.
In response, the joint research team systematically analyzed the structural characteristics of amyloid beta proteins and designed a peptide inhibitor to prevent self-assembly caused by misfolded structures.
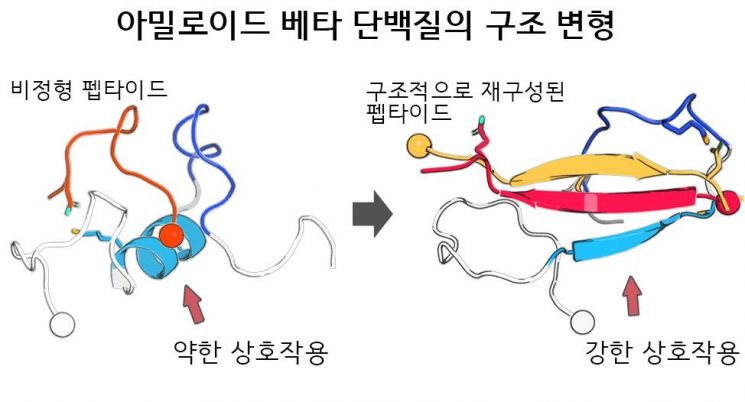 Schematic diagram of enhanced amyloid beta protein binding and inhibition of protein aggregation through structural reconstruction of non-structured peptides. Provided by National Research Foundation of Korea
Schematic diagram of enhanced amyloid beta protein binding and inhibition of protein aggregation through structural reconstruction of non-structured peptides. Provided by National Research Foundation of Korea
First, to inhibit aggregation, a high concentration of peptide aggregation inhibitor must be present in greater amounts than the amyloid beta protein. In addition, structural changes are required to form a stable complex.
Most importantly, for aggregation inhibition to be effective, the shapes of the inhibitor and the target must fit together well, like a key fitting into a lock. However, both amyloid beta proteins and previously developed peptide aggregation inhibitors have unstructured forms, resulting in weak binding between them.
To address this, the joint research team induced the formation of an antiparallel beta-sheet structure, enabling stable complex formation with amyloid beta proteins in their unstructured state.
As a result, the formation of pathogenic fibrillar aggregates of amyloid beta protein was reduced compared to previously developed inhibitors, and cellular toxicity was significantly alleviated.
Additionally, the joint research team explained that the inhibitor demonstrated suitable performance for therapeutic and preventive applications in tests evaluating its ability to cross the blood-brain barrier in vitro and its stability in plasma.
Professor Jungon Kim stated, "The joint research team has identified the structural characteristics of amyloid beta proteins and proposed a rational design method for peptides capable of forming stable complexes. This technology will serve as an important foundation for advancing research into therapeutics for Alzheimer's disease."
This research was supported by the Mid-Career Researcher Program, the Postdoctoral Domestic Fellowship, and the Sejong Science Fellowship, all funded by the Ministry of Science and ICT and the National Research Foundation of Korea. The research results were published on May 22 in the international chemistry journal 'Angewandte Chemie International Edition.'
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.










