STCube, a precision medicine-based immuno-oncology drug development company, announced on April 30 that it presented its next-generation immuno-oncology treatment strategies and clinical research results targeting the BTN1A1-YAP1 axis at the '2025 American Association for Cancer Research Annual Meeting (AACR 2025)' held in Chicago, USA.
This presentation is significant in that it proposed the potential for a new immuno-oncology mechanism that can overcome the limitations of existing immunotherapies. STCube has elucidated the interaction mechanism between BTN1A1 and YAP1. The company has provided empirical evidence that a therapeutic strategy targeting these molecules can help overcome anti-cancer drug resistance and improve treatment response in colorectal and lung cancers.
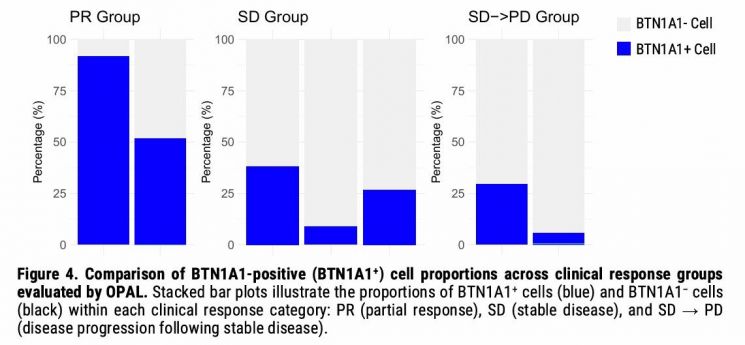
The first poster, titled 'BTN1A1 and YAP1 Expression as Biomarkers in Colorectal Cancer and Results from the Nelmastobart Phase 1b Study,' focused on the correlation between BTN1A1 expression rates and clinical response in a phase 1b investigator-initiated study involving patients with metastatic colorectal cancer.
This investigator-initiated clinical trial is a phase 1b/2 study evaluating the efficacy and safety of a combination therapy of Nelmastobart and Capecitabine as a third-line or later treatment for metastatic colorectal cancer. In the phase 1b study, 12 patients with microsatellite stable (MSS) colorectal cancer were enrolled over seven months. Among the 12 patients, there were 2 partial responses (PR) and 10 stable diseases (SD), resulting in an objective response rate (ORR) of 17% and a disease control rate (DCR) of 100%. Subsequently, a phase 2 trial involving 39 patients was conducted at doses of Nelmastobart 800 mg/m² and Capecitabine 1000 mg/m². The study is currently in the follow-up phase.
When the seven phase 1b patients were categorized by clinical response (PR, SD, SD→PD), the combination therapy with Nelmastobart showed a significant survival benefit in the group with high BTN1A1 expression. The partial response (PR) group had a markedly higher BTN1A1 expression rate compared to the stable disease (SD) group or the group whose disease progressed after stable disease (SD→PD), and a decreasing trend in BTN1A1 expression was observed moving toward the latter groups.
Yoo Seunghan, Chief Scientific Officer (CSO) of STCube, stated, "Even in the SD group, tumor size reduction was observed, and meaningful treatment responses were seen in about 45% of all phase 1b patients." He added, "The YAP1 high-expression group accounted for 50% of all patients and showed a close correlation with BTN1A1-positive response. Compared to the low-expression group, the high-expression group demonstrated superior progression-free survival (PFS) outcomes."
He continued, "These results are early clinical data confirming the potential of a precision biomarker-based treatment strategy prior to full-scale clinical entry in colorectal cancer." He visually demonstrated through OPAL (multiplex immunofluorescence staining) analysis that BTN1A1 and YAP1 are highly co-expressed, especially in colorectal cancer. He further explained, "BTN1A1 interacts with YAP1, a key regulator in the Hippo signaling pathway involved in cancer cell growth, immune evasion, and drug resistance, placing it at the center of the complex mechanisms underlying anti-cancer drug resistance."
The second poster, titled 'Therapeutic Targeting of the BTN1A1-YAP1 Pathway as a Novel Mechanism of Cancer Immune Evasion,' investigated the functional relationship between BTN1A1 and YAP1 in colorectal and lung cancer cell lines through 3D spheroid experiments. The STCube research team demonstrated that co-expression of these two proteins is a major mechanism of anti-cancer drug resistance and proposed a new dual-targeted therapeutic strategy that inhibits this pathway via BTN1A1.
In 3D spheroid experiments according to YAP1 expression status, Nelmastobart showed a strong synergistic effect when combined with existing anti-cancer drugs TAS-102 (for colorectal cancer) and Docetaxel (for lung cancer). Notably, therapeutic efficacy was confirmed even in resistant models with YAP1 overexpression. Inhibition of BTN1A1 significantly reduced YAP1-related drug resistance and simultaneously activated T cell-mediated immune responses, demonstrating a complex mechanism of action.
CSO Yoo stated, "This presentation suggests that BTN1A1 is not merely a novel immune checkpoint protein, but a key target and complex biomarker that can simultaneously regulate cancer immune evasion and anti-cancer drug resistance." He added, "By demonstrating the value of BTN1A1-targeted therapy through actual clinical and spheroid data, this has become an opportunity to underscore the potential success and competitiveness of Nelmastobart's clinical development strategies for colorectal and lung cancers going forward."
STCube has received approval from the Ministry of Food and Drug Safety for a phase 1b/2 clinical trial plan (IND) for the development of Nelmastobart, TAS-102, and Bevacizumab triple combination therapy for the treatment of metastatic/recurrent colorectal cancer. The combination of TAS-102 and Bevacizumab is known as the most effective global standard therapy among third-line or later treatments for metastatic colorectal cancer. In this clinical trial, after determining dose-limiting toxicity (DLT) in phase 1b, only patients with BTN1A1-positive expression will be selected for phase 2, making it a precision biomarker-based study. Full-scale patient dosing is scheduled to begin within the first half of the year.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.