Essential Pump-Circulated Stack Structure Without Expensive Ion Exchange Membrane,
Simply Designed with a 3D Printer
Globally, as the adoption of renewable energy increases, the demand for Energy Storage Systems (ESS) is also rising. However, concerns have emerged regarding fire hazards, high costs, and environmental issues.
In this context, a research team at Pusan National University has attracted attention by announcing research results that increased the reversibility and lifespan of batteries by using a simple design with a 3D printer, utilizing easily accessible and low-cost zinc and manganese, and employing eco-friendly amino acids as additives. The emergence of this safer, more affordable, and efficient next-generation technology is expected to accelerate the era of large-scale power storage linked to renewable energy.
Pusan National University (President Choi Jaewon) announced on the 10th that Professor Park Minjun's research team from the Department of Nano Energy Engineering has developed a new 'long-life, low-cost zinc-manganese redox flow battery' for next-generation ESS (Energy Storage Systems) by removing the ion exchange membrane, which accounts for 40% of the cost of redox flow batteries.
 Professor Minjun Park, Professor Junhee Kang.
Professor Minjun Park, Professor Junhee Kang.
The ion exchange membrane is a polymer membrane that selectively allows the passage of ions (protons/hydrogen ions) to facilitate the operation of redox flow batteries.
Recently, as the demand for ESS has been increasing, there have been continuous fire accidents in lithium-ion batteries, which are commonly used, leading to ongoing efforts to find alternatives or enhance safety.
As an alternative, redox flow batteries are being actively developed and are attracting attention as secondary batteries suitable for ESS due to their ability to independently design capacity and output, as well as their long lifespan and safety.
Redox flow batteries are a type of rechargeable battery that converts chemical energy into electrical energy. They are particularly useful in fields requiring large-scale energy storage. When used with solar and wind power generation, they help stabilize power supply, enable efficient operation by adjusting supply during peak hours, and provide long-term power supply to hospitals, data centers, and more.
While conventional lithium-ion batteries can overheat and catch fire due to heat buildup during reactions at solid electrodes, and repeated charging and discharging can damage electrodes, increasing the risk of short circuits, redox flow batteries use liquid electrolytes, which prevent overheating and explosion risks. The chemical reactions occur in the electrolyte solution, so the electrodes are not damaged.
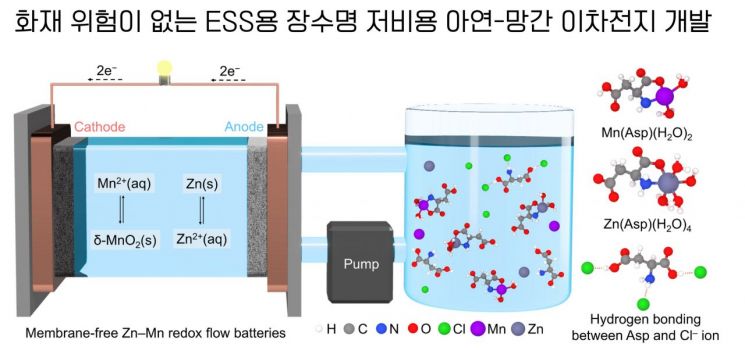
However, the high cost of redox flow batteries has limited their commercialization. Although there have been previous studies on 'redox flow batteries without ion exchange membranes', which account for over 40% of the cost, there has been a lack of systematic research on pump-circulated stack structures for large-scale applications.
Redox flow batteries contain electrolytes. While general batteries have a fixed, box-like shape where reactions occur inside, redox flow batteries have a structure where the liquid (electrolyte) continuously flows and reacts. Efficient circulation of this electrolyte is crucial, and the core technology is the 'pump-circulated stack structure'.
The electrolyte is stored in a large separate tank, and a pump sends the electrolyte into the battery to induce chemical reactions that generate electricity. The reacted electrolyte then returns to the tank for reuse. As the electrolyte continuously circulates, it maintains uniform reactions, and replacing the electrolyte allows for easy renewal. By increasing the size of the electrolyte tank without enlarging the battery itself, capacity can be scaled up for large-scale storage.
However, previous studies could not solve the problem of electrolyte mixing and electrode instability during pump circulation without ion exchange membranes, making commercialization difficult.
This research implemented a pump-circulated stack structure?previously unrealized in membrane-less redox flow batteries?using a separator designed with a 3D printer.
Additionally, instead of the widely used and expensive cation exchange membrane and vanadium electrolyte in conventional vanadium-based redox flow batteries, the team used low-cost zinc and manganese.
Zinc and manganese are key materials for the anode and cathode of redox flow batteries. Zinc donates electrons (anode) and manganese receives electrons (cathode) to generate electricity. Compared to vanadium, they are less expensive and more stable, reducing fire risk.
The research team applied an eco-friendly, multifunctional amino acid additive to the electrolyte, which acts on both the anode and cathode, thereby increasing both the lifespan and capacity of the battery.
As a result, the zinc-manganese redox flow battery achieved an energy density ten times higher than previous studies for batteries of the same capacity, marking a tenfold improvement in battery performance.
Professor Park Minjun from the Department of Nano Energy Engineering at Pusan National University stated, "This research will be a significant leap forward for the widespread adoption of safe ESS without fire risks," and added, "It is expected to bring us closer to the commercialization of eco-friendly aqueous redox flow batteries."
This study, which implemented a membrane-less zinc-manganese redox flow battery using a 3D-printed design to create an ultra-low-cost stack and significantly improved battery lifespan and capacity using an eco-friendly amino acid additive (aspartic acid), was published in the March 3rd issue of the international journal 'Advanced Energy Materials' (Impact Factor 24.4, top 2.9% in the energy field).
This research was jointly conducted by Professor Park Minjun's team and Professor Kang Junhee's team from the Department of Nano Energy Engineering at Pusan National University, and Professor Oh Pilgeon’s team from National Pukyong University, with support from the Excellent Young Researcher Program of the Ministry of Science and ICT and the National Research Foundation of Korea in 2024.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.