Precise Control of MOF Pores via Ion Exchange
First Application to Deuterium Separation
Separation Efficiency Doubled and Published in Angew. Chem. Int. Ed.
A technology has been developed to control the pores of porous materials at the 0.01 nanometer (10-9m) scale.
Using this technology, it is possible to efficiently separate deuterium, which is difficult to separate due to its similarity in properties to regular hydrogen and its presence at only 0.015% of all hydrogen in nature. Deuterium, an isotope of hydrogen, is a key resource that can be used in nuclear fusion power generation, semiconductor processes, and more.
Professor Hyun-Chul Oh’s team from the Department of Chemistry at UNIST and Professor Eun-Sung Lee’s team from the Department of Chemistry at Seoul National University announced on the 12th that they have succeeded in controlling the pores of a porous material, metal-organic frameworks (MOFs), down to the 0.01 nanometer scale through an ion-exchange method.
 Oh Hyun-chul, Professor at UNIST.
Oh Hyun-chul, Professor at UNIST.
They also explained that this ultra-fine control nearly doubled the efficiency of deuterium separation in the metal-organic frameworks.
The research results were published on the 12th of last month in the world-renowned scientific journal Angewandte Chemie International Edition (IF 16.1), recognizing its significance.
 Professor Eunseong Lee, Seoul National University.
Professor Eunseong Lee, Seoul National University.
By utilizing the pores of the porous new material metal-organic frameworks, it is possible to separate deuterium from hydrogen. To increase separation efficiency, the pore size, which acts as a sieve, must be precisely matched. Since both hydrogen and deuterium have pore sizes at the level of about 0.3 nanometers, ultra-precise control at the 0.01 nanometer level is required.
The joint research team succeeded in adjusting the pore entrance size of the metal-organic framework material JCM-1 by exchanging ions from nitrate (NO₃?) ions to chloride (Cl?) ions, changing the pore entrance size from approximately 0.39 nanometers to 0.36 nanometers.
The team analyzed that chloride ions pull the external framework connected to the pores inward more strongly than nitrate ions, resulting in a change in the pore entrance size.
The JCM-1(Cl?) with the reduced pore entrance showed a selectivity for deuterium separation that increased from 14.4 to 27.7, nearly doubling compared to JCM-1(NO₃?), which did not have the reduced pore size.
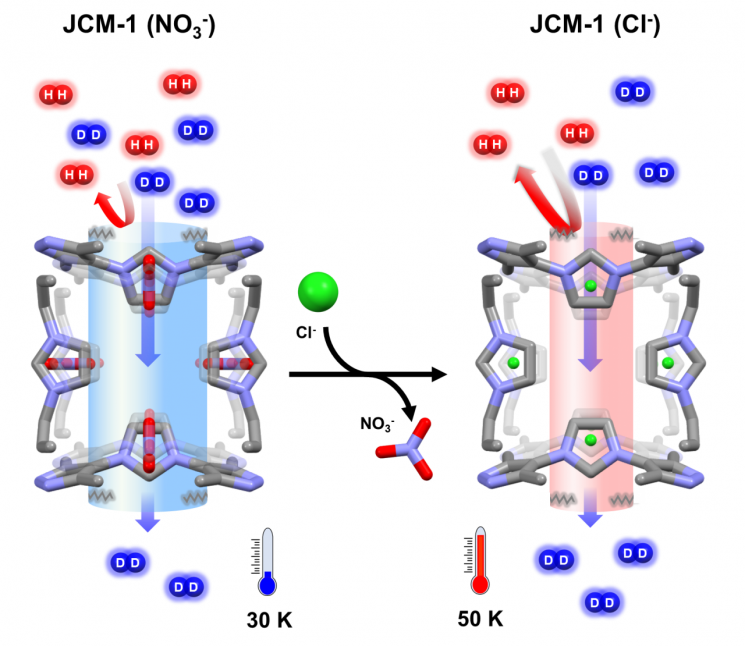 Schematic diagram of pore control and deuterium separation of porous materials through ion exchange method.
Schematic diagram of pore control and deuterium separation of porous materials through ion exchange method.
Even JCM-1(NO₃?) showed more than nine times better selectivity compared to the conventional cryogenic distillation method performed at 24K (-249.15°C). The selectivity of JCM-1(Cl?) increased by about 18 times compared to the conventional cryogenic distillation method. JCM-1 is a material developed by Professor Eun-Sung Lee’s team.
 Researcher Kim Hyun-rim.
Researcher Kim Hyun-rim.
First author researcher Hyun-Rim Kim explained, “JCM-1(Cl?) maintains stable performance even at a relatively higher temperature of 50K (-223.15°C) compared to the conventional cryogenic distillation method at 24K (-249.15°C), showing potential for use in various industries such as nuclear fusion and semiconductor manufacturing.”
Professor Hyun-Chul Oh, who led the research at UNIST, stated, “This achievement presents a new method to precisely control the nanopore size of porous materials and can be applied not only to isotope separation but also to various gas separation fields.”
This research was conducted through mid-career and basic research projects supported by the Ministry of Science and ICT.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![From Bar Hostess to Organ Seller to High Society... The Grotesque Con of a "Human Counterfeit" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















