Mega Deal with Eli Lilly
Obesity Treatment Effect Proven in Australian Clinical Trial
Domestic new drug bio venture Olix has secured a mega contract worth over 900 billion won. The market for simultaneous treatment of metabolic dysfunction-associated steatohepatitis (MASH) and obesity is opening, and Olix has licensed a promising new drug candidate to Eli Lilly, which is well-known for obesity treatments.
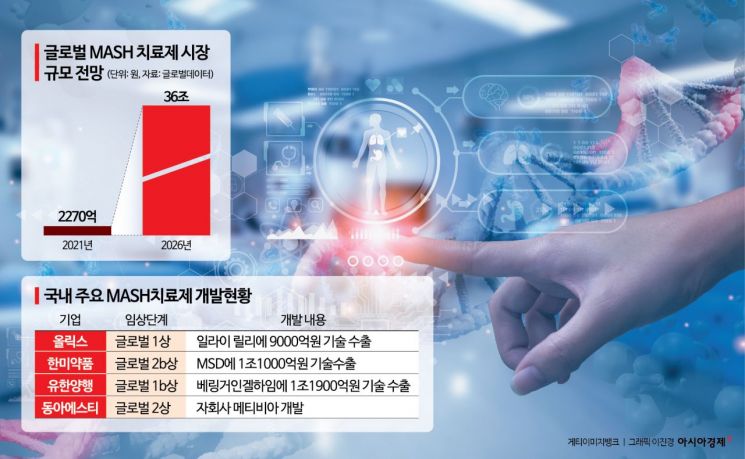
According to industry sources on the 11th, Olix recently signed a technology transfer agreement with Eli Lilly worth a total of $630 million (approximately 915 billion won), including an upfront payment and milestone payments upon achieving various stages of success. Olix received the upfront payment from Eli Lilly to complete Phase 1 clinical trials in Australia, while Eli Lilly will conduct further research and development and commercialization. This contract aims to develop and commercialize Olix’s ‘OLX702A,’ a Phase 1 clinical candidate for MASH and cardiovascular/metabolic disease treatment. Olix will grant Eli Lilly an exclusive license for this clinical candidate. In 2015, Eli Lilly also signed a technology transfer agreement worth $700 million (approximately 1.0167 trillion won) with Hanmi Pharmaceutical for an immunological disease treatment.
MASH occurs when excessive calorie intake causes excessive fat accumulation in the liver. The fat accumulated in liver cells oxidizes, producing toxicity in the fat and causing liver cell damage. Immune cells become excessively activated, producing inflammatory cytokines that cause the immune system to attack itself. This leads to inflammation and fibrosis in the liver, potentially resulting in serious liver diseases such as cirrhosis, liver cancer, and liver failure. Because liver disease progresses through multiple stages, it has been difficult to treat with single-target therapies. The global prevalence of MASH is 2-4%, with 3-5% prevalence in the United States.
Last March, Madrigal Pharmaceuticals in the U.S. became the first in the world to receive FDA approval for the use of ‘Resdefipra,’ marking the beginning of the market formation. Since Resdefipra is expensive and its effectiveness is limited, there is room for new drugs to enter the market. According to market research firm GlobalData, the MASH treatment market is expected to reach $25.3 billion (approximately 33.7375 trillion won) by 2026. It is estimated that there are currently about 440 million MASH patients worldwide.
MASH treatments can also be used as obesity treatments by regulating satiety and energy metabolism. The new drug candidate Olix licensed this time can simultaneously treat metabolic dysfunction-associated steatohepatitis and obesity. In the Phase 1 clinical trial conducted in Australia, about 60 subjects were followed for 84 days, resulting in an average reduction of about 1 inch (2.5 cm) in abdominal circumference. Currently approved obesity treatments require weekly administration, but Olix’s new drug candidate is administered once every three months, offering superior patient convenience.
Minyong Eom, a researcher at Shinhan Investment Corp., said, "Eli Lilly likely reviewed all MASH treatment candidates before signing the contract with Olix. Considering that Danish pharmaceutical company Novo Nordisk is developing a competing drug based on the same principle as Olix, there must have been a judgment that Olix’s ‘OLX702A’ surpasses competing drugs."
Currently, other domestic pharmaceutical companies are also developing MASH treatments. Hanmi Pharmaceutical is conducting a global Phase 2b trial of ‘Eposipegtrutide,’ a triple-action bio new drug that simultaneously activates glucagon, which increases energy metabolism in the body; GLP-1, which helps insulin secretion and appetite suppression; and the ‘GIP receptor,’ which promotes insulin secretion and has anti-inflammatory effects. MetaVia, a subsidiary of Dong-A ST, is conducting a Phase 2 trial of the MASH treatment candidate ‘DA-1241.’ They have announced that the analysis of the Phase 2 topline data confirmed efficacy and safety.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.