SK Biopharm recorded an operating profit of 96.3 billion KRW last year, marking its highest-ever profit.
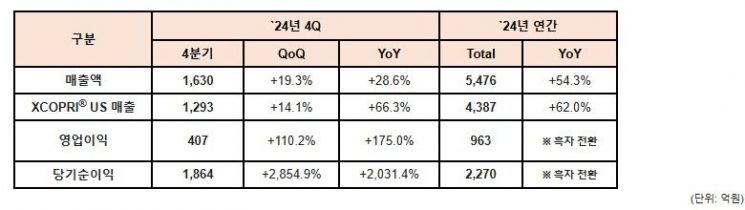
On the 6th, SK Biopharm announced that it posted sales of 547.6 billion KRW and an operating profit of 96.3 billion KRW last year. Sales increased by 54.3% compared to the same period the previous year, and operating loss of 37.5 billion KRW turned into a profit.
Sales of Cenobamate, an epilepsy drug, in the U.S. reached approximately 438.7 billion KRW, growing 62.0% year-on-year and surpassing the upper end of the guidance (target) set last year, demonstrating solid growth.
Regarding this sales achievement, SK Biopharm stated, "It is meaningful as the first annual profit achieved solely through the growth of Cenobamate sales without the help of one-time revenues such as milestones," adding, "Last year was an important milestone for the commercialization of innovative new drugs in South Korea."
Last year, SK Biopharm strengthened its specialty sales organization and personnel, including epilepsy centers and dedicated long-term care staff for patients. This year, to expand touchpoints with patients, the company plans to implement an aggressive marketing strategy, including its first-ever DTC (Direct-to-consumer) advertising.
Cenobamate is expanding its presence not only in the U.S. market but also globally. Strong sales in the global market have proven the potential for innovative new drug commercialization, with the total cumulative number of prescribed patients exceeding 140,000 last year. Additionally, starting with the New Drug Application (NDA) submission in Brazil, SK Biopharm is advancing into about 17 Latin American countries. The submission of the NDA in China, which secured milestone revenue, also contributed to last year's strong sales. Based on successful clinical results in the three Northeast Asian countries (South Korea, China, and Japan), partner companies in South Korea and Japan will follow China's lead in proceeding with country-specific approval application procedures.
SK Biopharm is also aiming to expand the market through indication and age range extensions for Cenobamate. Within this year, the company plans to secure top-line results from Phase 3 clinical trials for indication expansion from partial seizures to generalized tonic-clonic seizures (PGTC) and develop an oral suspension formulation to facilitate administration to pediatric patients, followed by NDA submission.
Along with the growth of Cenobamate, SK Biopharm plans to maximize the leveraging effect of the sales network and marketing platform established through direct sales in the U.S. and seek new growth engines. The company aims to achieve visible results for follow-up commercialization products of Cenobamate within the first half of this year.
Through the development of RPT (Radiopharmaceutical Therapy) and TPD (Targeted Protein Degradation) selected as next-generation new modalities, as well as expansion of research and development (R&D) capabilities in the small molecule field, SK Biopharm plans to diversify its portfolio.
In the RPT field, the company acquired the candidate substance 'FL-091' (currently SKL35501) from Fullife Technology and secured a stable supply of radioactive isotopes (RI) through a supply contract for Actinium-225 (Ac-225) with Therapaower. Furthermore, to continuously discover candidate substances and conduct in-house research and development, SK Biopharm has signed joint research agreements with various companies and organizations such as the Korea Institute of Radiological & Medical Sciences and ProTherapeutics, accelerating comprehensive business expansion with the goal of becoming a global leading RPT player.
In the TPD field, SK Biopharm is focusing on discovering and developing protein degraders based on MOPED™, an innovative platform for molecular glue (MG) discovery, through SK Life Science Labs.
Additionally, SK Biopharm plans to expand its R&D capabilities beyond the central nervous system (CNS) field into oncology by developing new pipelines related to cancer and Parkinson’s disease in the small molecule field, where it already has strengths.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.