Partial Amendment Notice for the "Regulations on New Medical Technology Assessment"
The government will expand the early market entry of new medical technologies by prioritizing their use in the field first, followed by evaluations of safety and efficacy through an advanced entry system.
On the 28th, the Ministry of Health and Welfare announced that it will publicly notify the partial amendment of the "Regulations on the Evaluation of New Medical Technologies" until December 9. The amendment includes strengthening safety management of advanced entry medical technologies, establishing regulatory grounds for re-evaluation of new medical technologies, and extending the evaluation deferral period for certain technologies.
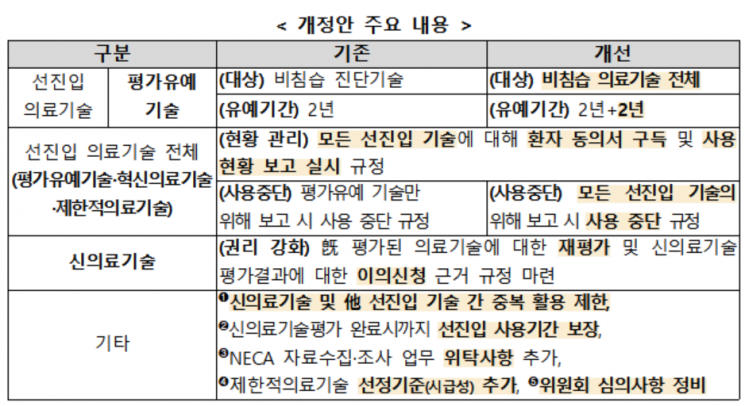
The Ministry of Health and Welfare has continuously improved the advanced entry system for new medical technology evaluations to enable early utilization of outstanding medical technologies in patient treatment. However, due to the nature of advanced entry technologies whose new medical technology evaluations have not been completed, there have been consistent calls to extend the advanced entry period (evaluation deferral of 2 years) to strengthen safety management during use and to generate sufficient clinical evidence.
Accordingly, since last year, the Ministry has incorporated innovative measures identified in the Biohealth New Market Creation Strategy Meeting and the Regulatory Innovation Strategy Meeting, as well as discussions from the public hearing on improving the advanced entry-post evaluation system, to promote amendments to the regulations aimed at simultaneously enhancing the safety of advanced entry technologies and revitalizing market entry for companies.
The amendment mandates patient consent forms and usage status reports when using advanced entry technologies to strengthen the safety management system. Additionally, advanced entry technologies deemed to have a high risk level by the evaluation committee will be subject to suspension of use, thereby removing technologies with safety issues. Provisions for re-evaluation of medical technologies will be established to prepare for cases where there are concerns about safety or changes in efficacy, and periodic management of the usefulness and value of technologies will also be promoted.
Furthermore, to activate market entry of advanced entry technologies, the scope of evaluation deferral has been expanded to cover all non-invasive medical technologies. The usage period for evaluation deferral technologies will be extended up to a maximum of 4 years (with one extension), and advanced entry medical technologies currently under new medical technology evaluation application can continue to be used until results are available, supporting continuous clinical utilization.
Kim Guk-il, Director of Health and Medical Policy at the Ministry of Health and Welfare, stated, "Since the amendment of the 'Regulations on the Evaluation of New Medical Technologies' in 2022, we have prepared this system improvement plan by reflecting opinions raised by the medical community, industry, and civic groups, as well as difficulties encountered in the field. We expect that while strengthening safety management of advanced entry medical technologies, excellent technologies will be rapidly utilized in the market."
Detailed information on the amendment can be found on the Ministry of Health and Welfare website → Information → Laws and Regulations → Legislative/Administrative Notice Electronic Public Hearing, and related opinions can be submitted to the Ministry’s Medical Resources Policy Division by December 9.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















