Under current law, designation is possible for up to 20 items
but only 11 are handled due to suspension of some item production
"Prolonged medical crisis... necessary policy for consumers"
As the medical crisis triggered by the controversy over increasing the number of medical school admissions continues into its eighth month, calls to expand the range of over-the-counter drugs available at convenience stores are resurfacing. It is argued that this policy is essential for the public, who are consumers of medicines, especially during late-night hours and holidays when hospitals and pharmacies are closed.
According to the convenience store industry on the 29th, discussions on expanding the range of over-the-counter drugs at convenience stores have not taken place even once in the six years since 2018. A representative from the Korea Convenience Store Industry Association stated, "Now that the medical crisis has become a reality, it is time to revisit the expansion of over-the-counter drug items," adding, "The Ministry of Health and Welfare has remained inactive with no movement since the discussions in 2018, showing a state of inertia."
The over-the-counter drug sales system was introduced in November 2012 to alleviate the inconvenience of purchasing medicines during late-night and holiday hours. Accordingly, the sale of medicines was permitted only at stores open 24 hours a day, year-round, other than pharmacies. In the case of convenience stores, following the revision of the Pharmaceutical Affairs Act, sales began for a total of 13 items: 7 cold, fever, and pain relief medicines, 4 digestive aids, and 2 anti-inflammatory drugs.
 Over-the-counter medicines such as cold medicine are displayed at a convenience store.
Over-the-counter medicines such as cold medicine are displayed at a convenience store. [Photo by Asia Economy DB]
The industry has consistently pointed out that the expansion of over-the-counter drug items has not been realized to the extent stipulated by the Pharmaceutical Affairs Act. The current Pharmaceutical Affairs Act stipulates that the Minister of Health and Welfare designates up to 20 items, but the handled items remain fixed at 13. Moreover, due to the withdrawal of domestic production facilities by Korea Janssen, production of two types of Tylenol has been suspended, reducing the number of handled items to 11.
An industry official said, "Although over-the-counter drug sales account for only about 0.3% of convenience store sales, it has a strong public character in minimizing consumers' time and economic losses for minor illnesses," adding, "The expansion of items should be realized to reduce medical expenses, including lowering the costs of hospital, clinic, and pharmacy use for patients with mild illnesses or low-income groups."
Discussions on expanding the range of over-the-counter drugs have not been absent. From March 2017 to August 2018, the 'Over-the-Counter Drug Designation Review Committee' held six meetings to discuss item adjustment plans but failed to reach conclusions on item inspection and readjustment. Although the committee was decided to be formed last year, it has yet to be established.
The biggest obstacle is the opposition from the Korean Pharmaceutical Association, which cites concerns over drug misuse. They argue that patients who should visit emergency rooms might instead take medicines and worsen their condition. The Korean Pharmaceutical Association even held rallies in 2018, when the committee meetings were ongoing, to oppose the expansion of items.
However, the industry counters the association's claims by citing the 2020 Consumer Monitoring results from the Pharmaceutical Policy Research Institute, which found that adverse effects are minimal when medicines are taken with attention to dosage and usage. Regarding concerns about drug misuse, they argue, "Expansion can be done gradually starting with medicines that have low potential for misuse."
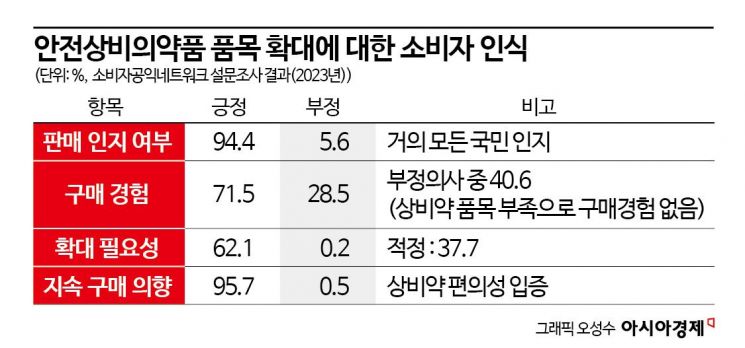
Consumers also share a consensus on expanding the range of over-the-counter drugs at convenience stores. According to a consumer awareness survey conducted last year by the Consumer Public Interest Network targeting 1,000 people, 62.1% of respondents who had experience purchasing over-the-counter drugs at convenience stores answered that "the number of items is insufficient and needs to be expanded."
Another industry official said, "The majority of the public feels the need to expand the number of over-the-counter drug items at convenience stores," adding, "It is urgent to expand consumer-demanded items with high safety such as rehydration salts, branch offices tax, and burn ointments, and to designate substitutes for items whose production has been discontinued."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














